The intermediates for the oxidation of isopropanol to acetone are shown. Calculate oxidation numbers for the indicated atoms, then use them to determine in which step oxidation occurs.
(e)
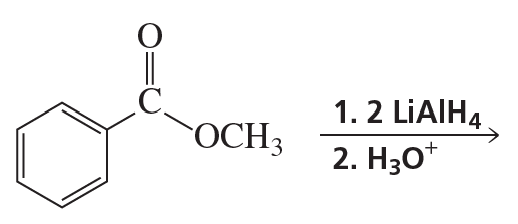
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: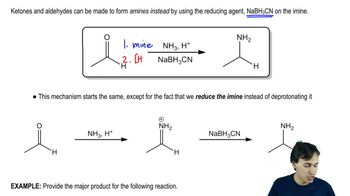
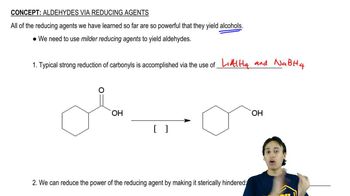
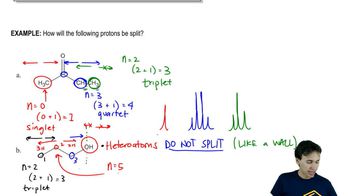

 6:02m
6:02mMaster General Features of Redox with a bite sized video explanation from Johnny
Start learning