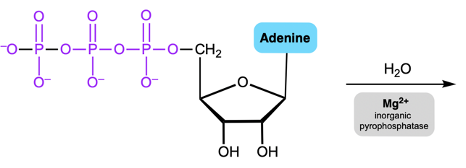
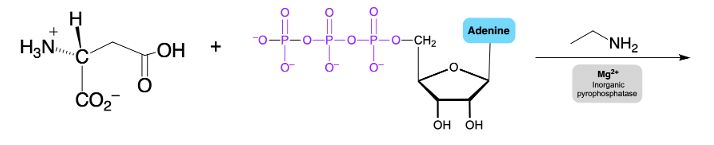
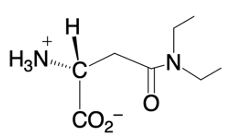
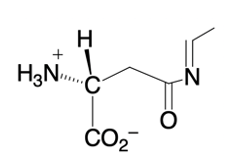
Phosphate anhydrides are important in biochemical reactions, particularly due to their multiple negative charges, which make the phosphorus atoms resistant to nucleophilic attack. A nucleophile, typically a negatively charged species, is less likely to approach a highly negative structure due to the repulsion between like charges. To facilitate nucleophilic attack on phosphate anhydrides, magnesium ions and a class of enzymes known as inorganic pyrophosphatases play a crucial role in reducing the overall negative charge.
The interaction between magnesium ions and phosphate anhydrides leads to the formation of a phosphate anhydride-magnesium complex. In this complex, magnesium forms ionic bonds with the phosphoryl oxygen atoms that are furthest from the R group of the anhydride. This bonding is represented by dotted lines, indicating that ionic bonds are not as strong as covalent bonds. By occupying the negative oxygens, magnesium effectively lowers the free negative charge on the surface of the phosphate anhydride.
As a result of this reduction in negative charge, the likelihood of a nucleophile attacking a phosphorus atom increases. The presence of magnesium, which can be visualized as a silver string, is essential in this process. This is similar to laboratory experiments involving magnesium strips, which also exhibit a silver color. Thus, when dealing with phosphate anhydrides, it is critical to first introduce magnesium along with inorganic pyrophosphatases to create a conducive environment for nucleophilic attack on the phosphorus atoms.