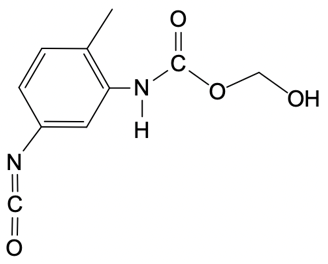
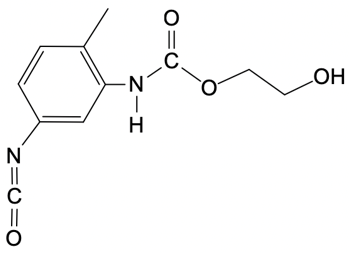
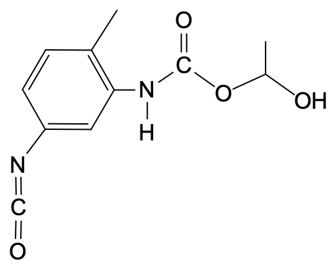
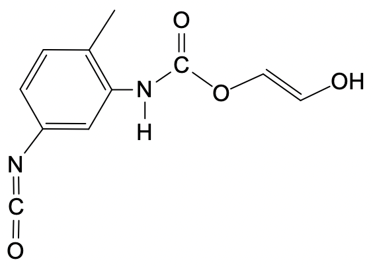
Polyurethanes are synthesized through a nucleophilic addition reaction involving a diol and toluene diisocyanate (TDI). The most common mixture of TDI used in this process consists of 80% 2,4-toluene diisocyanate and 20% 2,6-toluene diisocyanate. This means that when discussing TDI, the default reference is typically to the 2,4 isomer unless specified otherwise. The structure of toluene indicates that the methyl group is the priority substituent, leading to the designation of the 2 and 4 positions for the isocyanate groups.
In the formation mechanism of polyurethanes, two key steps are involved. The first step is the nucleophilic attack of the hydroxyl group from the diol on the electrophilic carbon of the isocyanate group. This reaction results in the formation of a carbamate intermediate. The second step involves the further reaction of this intermediate, leading to the formation of the polyurethane polymer. Understanding these steps is crucial for grasping how polyurethanes are formed and their applications in various industries.