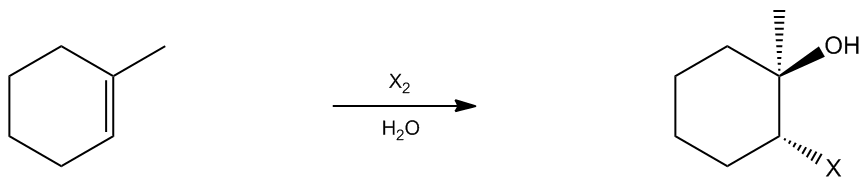
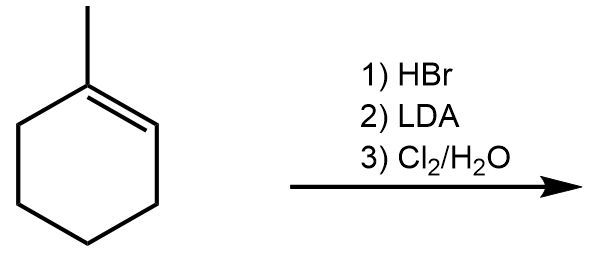
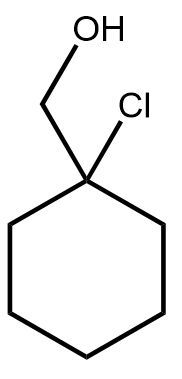
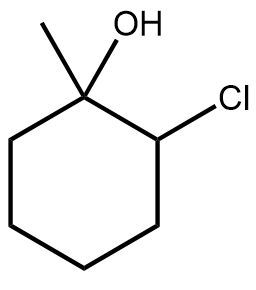
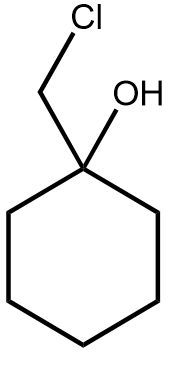
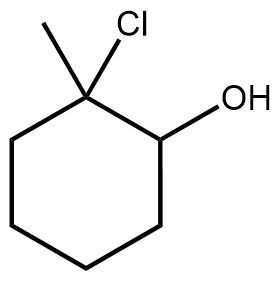
In the study of organic chemistry, halohydrin formation is a reaction that closely resembles halogenation but includes the presence of water. This reaction involves the addition of a diatomic halogen to a double bond in an aqueous environment, resulting in the formation of a halohydrin, which is characterized by the presence of both an alcohol and a halogen on the product molecule.
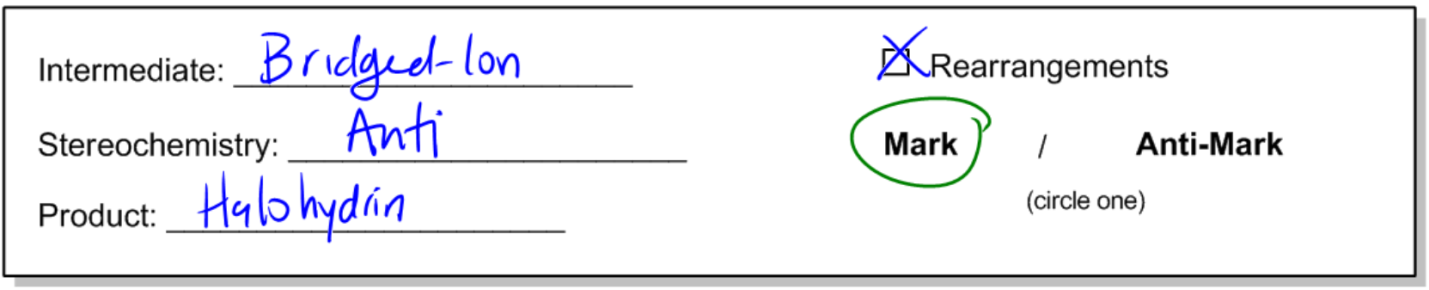
The general reaction can be represented as follows: when a double bond reacts with a diatomic halogen (e.g., Cl2 or Br2) in the presence of water, the outcome is a halohydrin. The mechanism begins with the formation of a bridged ion intermediate, similar to that seen in halogenation. However, the key distinction lies in the presence of water, which leads to the formation of an alcohol on one side of the molecule and a halogen on the other.
During this reaction, the stereochemistry of the product is anti, meaning that the halogen and the alcohol will be positioned on opposite sides of the molecule. This anti addition occurs due to the opening of the three-membered ring intermediate. Importantly, there are no rearrangements in this reaction because it does not involve the formation of a carbocation, which is typically a site for rearrangement in other reactions.
Halohydrin formation also follows Markovnikov's rule, which states that when adding two different substituents to a double bond, the more stable intermediate will dictate the regioselectivity of the reaction. In this case, the more stable product will have the halogen and the alcohol positioned according to the stability of the intermediates formed during the reaction.
In summary, halohydrin formation is a significant reaction in organic chemistry that highlights the interplay between water and halogens in the addition to alkenes, leading to the formation of halohydrins with specific stereochemical and regioselective properties.