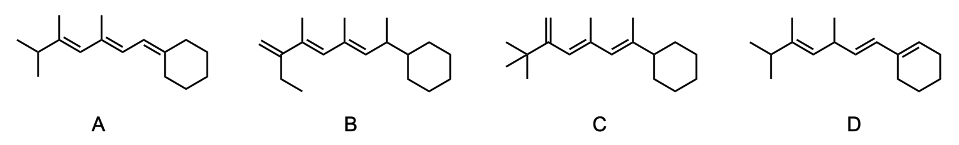
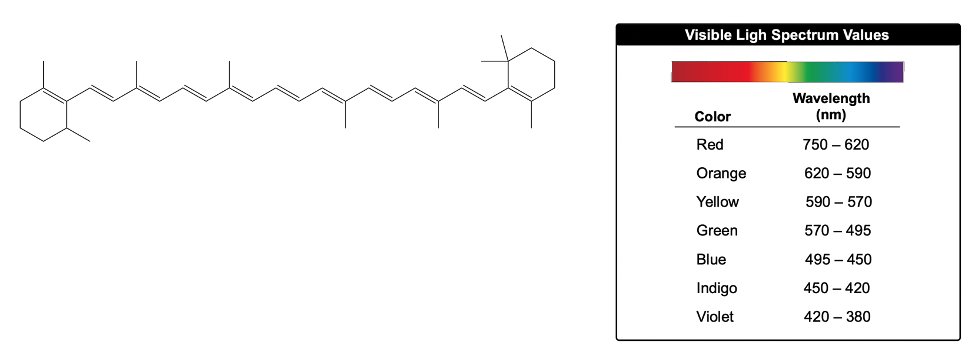
The Woodward-Pfizer rules provide a systematic approach to estimate the maximum wavelength of absorption, known as lambda max (λmax), for conjugated systems. These rules categorize the contributions to λmax into four main areas: the conjugated diene, alkyl oxochromic groups, exocyclic double bonds, and homoanular dienes.
Starting with the conjugated diene, it has a base λmax value of 217 nanometers. Each additional conjugated double bond increases λmax by 30 nanometers. For instance, if a conjugated diene has two additional double bonds, the calculation would be:
217 nm + (2 × 30 nm) = 277 nm.
Next, alkyl oxochromic groups, which are non-conjugated groups attached to the conjugated system, contribute an increase of 5 nanometers for each alkyl group. If there are four alkyl groups, the contribution would be:
4 × 5 nm = 20 nm.
Adding this to the previous total gives:
277 nm + 20 nm = 297 nm.
For exocyclic double bonds, which involve a double bond with one vanillic carbon as part of a ring and another extending outside, each contributes an additional 5 nanometers. If there are two such bonds, the contribution would be:
2 × 5 nm = 10 nm.
Thus, the total becomes:
297 nm + 10 nm = 307 nm.
Finally, homoanular dienes, which are conjugated dienes in the same ring in a cis configuration, add 39 nanometers each. If there is one homoanular diene, the contribution is:
39 nm.
Adding this to the previous total results in:
307 nm + 39 nm = 346 nm.
In summary, the Woodward-Pfizer rules allow for a structured estimation of λmax by considering the contributions from various structural features of the molecule. This systematic approach is essential for predicting the absorption characteristics of conjugated systems in organic chemistry.