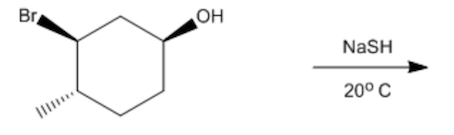
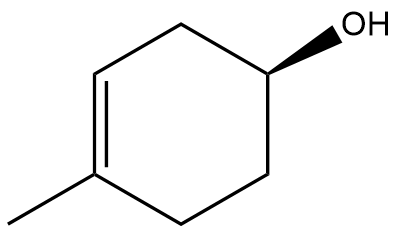
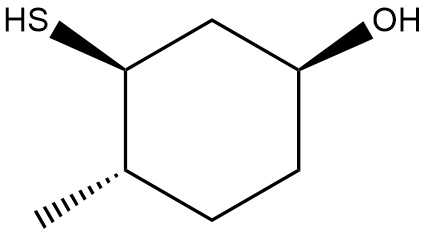
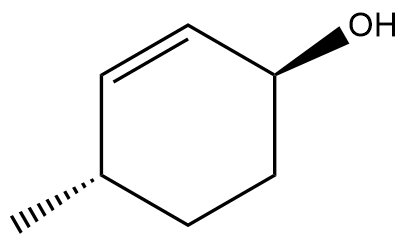
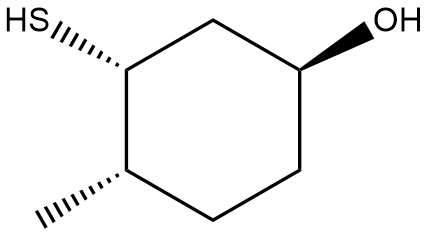
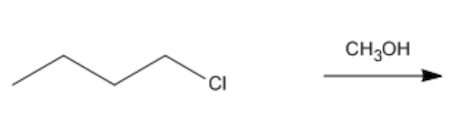
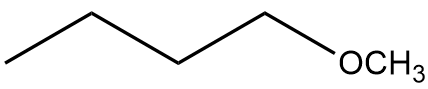
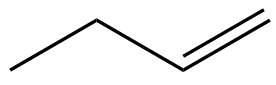
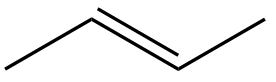
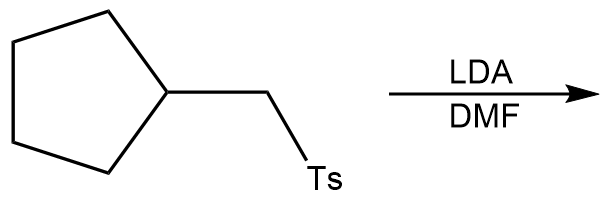
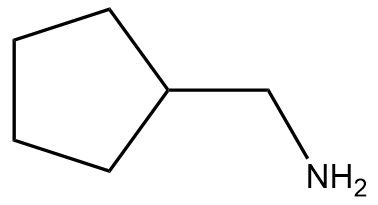
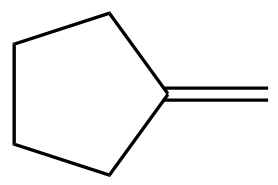
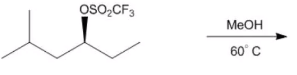
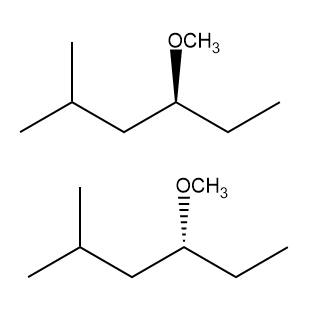
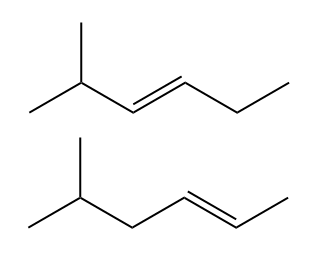
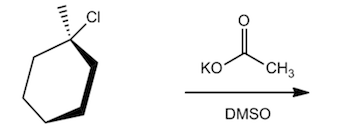
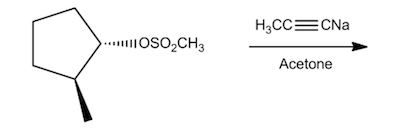In this section, we will integrate our understanding of four key chemical mechanisms and utilize a comprehensive flow chart to solve cumulative problems. The goal is to identify the appropriate mechanism based on the provided scenarios without explicit guidance. This exercise emphasizes the importance of recognizing the characteristics and outcomes of each mechanism, which are crucial for predicting the products of chemical reactions.
To effectively tackle these problems, it is essential to have a solid grasp of the mechanisms involved. Each mechanism has distinct features that dictate how reactants transform into products. Familiarity with these characteristics will enable you to navigate the flow chart confidently and make informed predictions about the reaction products.
As you work through the problems, focus on the following key aspects:
- Mechanism Identification: Use the flow chart to determine which mechanism applies to each problem. Understanding the flow of the chart is vital for accurate identification.
- Product Prediction: Based on your knowledge of the mechanisms, predict the products of the reactions. This requires a clear understanding of how each mechanism operates and the types of transformations it facilitates.
- Practice and Application: Engaging with these cumulative problems will reinforce your understanding and help solidify the concepts covered in this chapter.
By approaching these problems methodically, you will enhance your ability to analyze chemical reactions and apply your knowledge effectively. Let’s begin with the first problem and apply what we’ve learned!