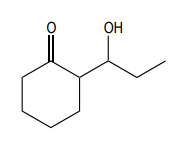
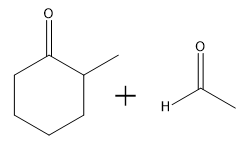
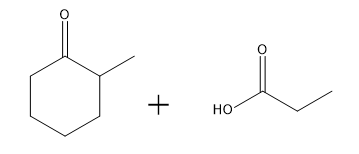
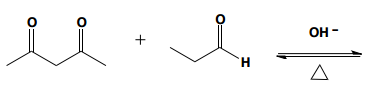
Aldol condensation is a significant reaction in organic chemistry, particularly when dealing with aldehydes and ketones. When attempting to perform an aldol condensation with a mixture of different aldehydes, the formation of mixed products is a common challenge. This occurs because each aldehyde can form its own enolate, leading to a variety of potential products. For instance, if aldehyde A can form an enolate at one position and aldehyde B at another, several reactions can take place, resulting in a complex mixture of products.
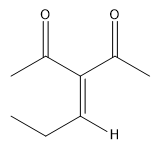
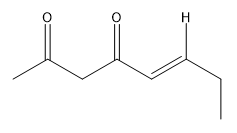
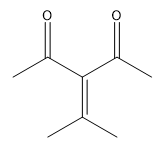
In a crossed aldol reaction, the enolate from one aldehyde can react with itself or with the other aldehyde, leading to products such as A + A, A + B, B + A, and B + B. Each of these combinations yields different compounds, which can be represented in a Punnett square format to visualize the potential outcomes. However, this approach is often impractical in synthetic chemistry due to the resulting mixture of products, which can be synthetically useless.
To illustrate, consider the following combinations: - The reaction of A with B produces a compound with a total of five carbons, combining the carbon skeletons of both aldehydes.- The self-condensation of A (A + A) results in a six-carbon product, while B + B yields a four-carbon compound. - Notably, A + B and B + A, although they contain the same number of carbons, are distinct compounds due to the different arrangements of the carbon atoms.
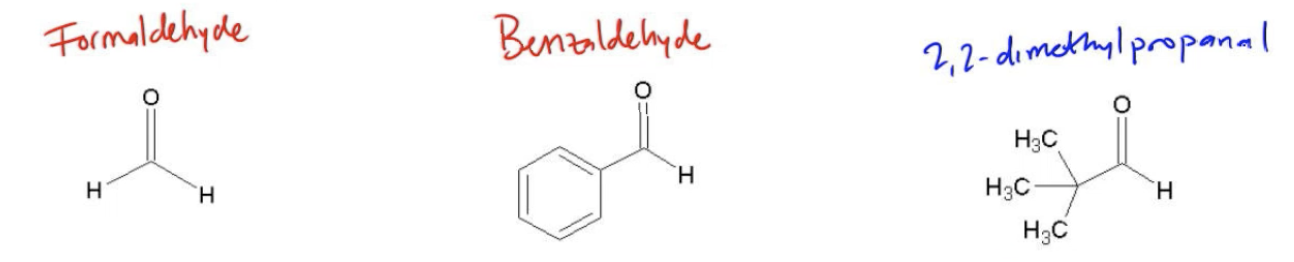
To avoid the complications of mixed products in aldol reactions, it is advisable to use at least one non-enolizable aldehyde or ketone. A non-enolizable compound lacks an alpha proton, which means it cannot form an enolate. Examples of such compounds include formaldehyde and benzaldehyde. By ensuring that one reactant cannot form an enolate, the reaction simplifies, allowing for a more predictable outcome where the enolate will only react with the non-enolizable aldehyde, leading to a single product.
In summary, understanding the dynamics of aldol condensation reactions, particularly in mixed systems, is crucial for effective synthetic strategies. By carefully selecting reactants, chemists can minimize the formation of unwanted byproducts and achieve desired outcomes more efficiently.