In electrophilic aromatic substitution (EAS) reactions involving ortho-para directing groups, the products typically consist of a mixture of ortho and para isomers. However, understanding the competition between these two positions can provide insights into predicting the major product. The ortho position has two reactive sites, while the para position has one, which might suggest a higher yield of ortho products. Yet, steric hindrance plays a crucial role; the para position is generally less hindered due to its distance from the electron-donating group, making it more favorable for substitution.
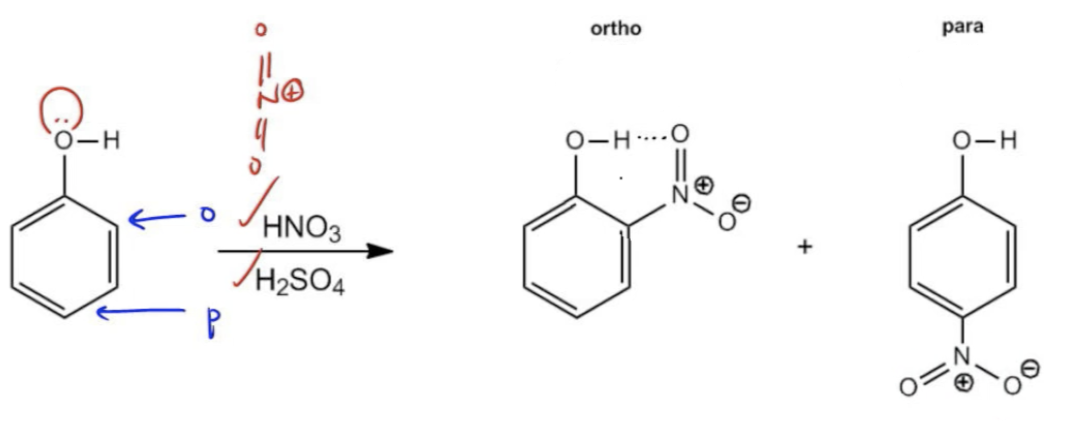
When asked to identify a single major product, the para product is often preferred due to this steric advantage. For example, in the sulfonation of toluene using sulfur trioxide and fuming sulfuric acid, the para sulfonic acid emerges as the major product despite the presence of two ortho positions. This trend holds true unless the final product can engage in intramolecular hydrogen bonding, which can favor the ortho position slightly. An example of this is the nitration of phenol, where the ortho product is favored due to the potential for hydrogen bonding between the hydroxyl and nitro groups, resulting in a ratio of approximately 60% ortho to 35% para.
In summary, while the general rule is that the para product is favored in ortho-para directing reactions due to steric hindrance, exceptions exist when hydrogen bonding can influence the outcome. Understanding these dynamics is essential for predicting the products of EAS reactions accurately.