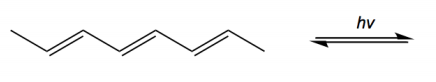
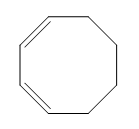
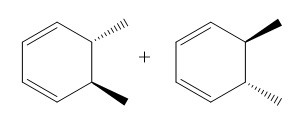
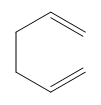
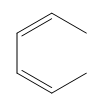
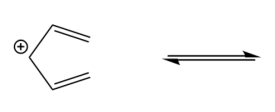
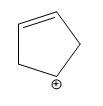
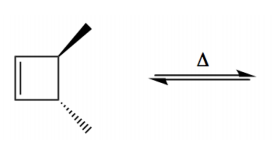Electrocyclic reactions are a fascinating area of organic chemistry that involve the conversion of conjugated systems into cyclic structures through the application of heat (thermal activation) or light (photochemical activation). Understanding the stereochemistry of these reactions is crucial, and there are systematic approaches to determine whether the reaction will proceed via a conrotatory or disrotatory mechanism.
The first step in analyzing an electrocyclic reaction is to determine the type of rotation. This can be done by assessing the number of pi bonds in the system and the type of activation. For instance, if you have an even number of pi bonds and are using thermal activation, the reaction will proceed via a conrotatory mechanism. Conversely, if the number of pi bonds is odd under thermal activation, the mechanism will be disrotatory. A helpful mnemonic to remember this is "etcetera" (e.t.c.), where 'e' stands for even, leading to conrotatory, and any change in the number of pi bonds or activation type will guide you to the opposite conclusion.
Once the type of rotation is established, the next step is to determine the stereochemistry of the product. This involves visualizing the three-dimensional structure of the molecule and assessing the spatial arrangement of substituents. The stereochemistry can be classified as either cis or trans based on the rotation type and whether the substituents are the same or different. A useful phrase to remember is "if it's the same, dis is sis," indicating that if the substituents are the same and the mechanism is disrotatory, the product will be cis. If the substituents differ or if the mechanism is conrotatory, the product will be trans.
In summary, mastering the determination of stereochemistry in electrocyclic reactions involves understanding the interplay between the number of pi bonds, the type of activation, and the spatial arrangement of substituents. By applying these principles and utilizing mnemonic devices, students can simplify the process and enhance their understanding of these complex reactions.