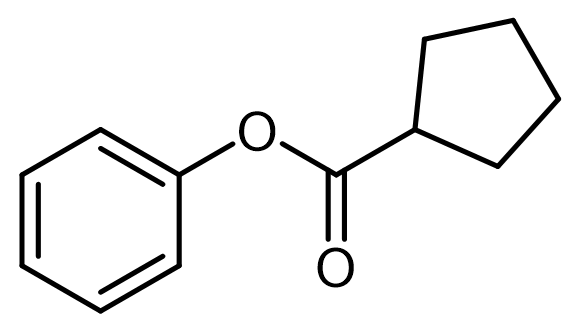
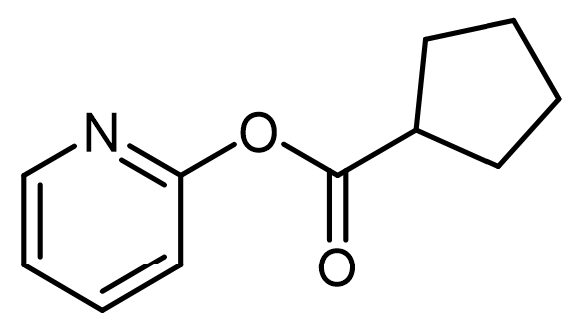
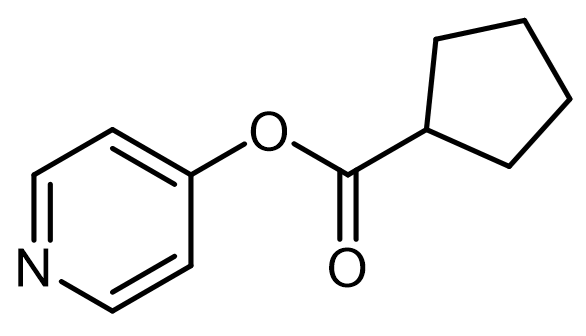
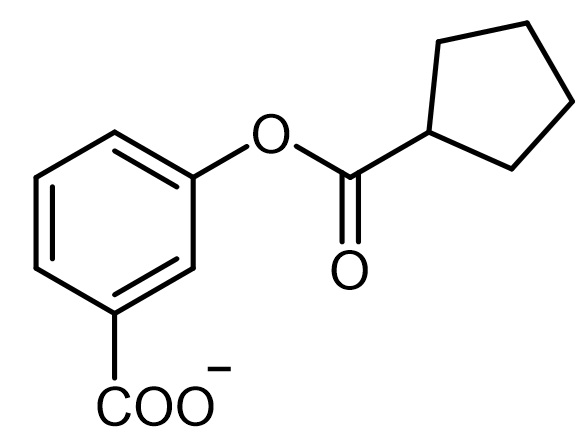
Intramolecular acid-base catalysis involves a catalyst, which can be an acid, base, or nucleophile, that is located within the reacting molecule itself. This type of catalysis is particularly significant in organic reactions where the proximity of functional groups can enhance reaction rates and selectivity.
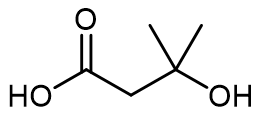
In the context of intramolecular general acid catalysis, an acidic group can facilitate reactions through intramolecular protonation. For instance, a carboxylic acid can serve as an effective acidic group. When present in a molecule, the carboxylic acid can promote the formation of an ester by enabling the interaction between the carboxylic acid and an alcohol within the same molecule. This interaction leads to the successful synthesis of the ester product.
Understanding the role of intramolecular catalysts is crucial for predicting reaction mechanisms and optimizing conditions for desired chemical transformations. The ability of an acidic group to enhance reactivity through protonation exemplifies the importance of molecular structure in influencing chemical behavior.