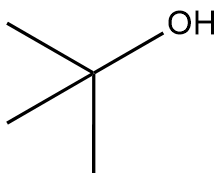
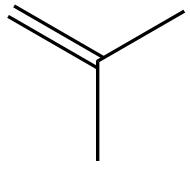
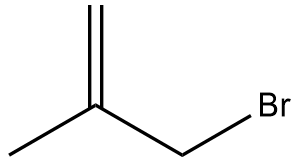
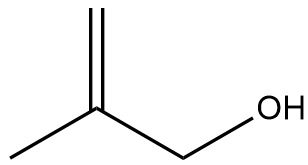
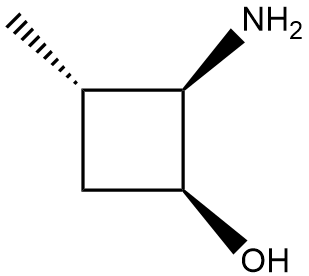
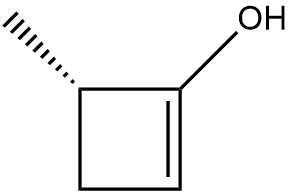
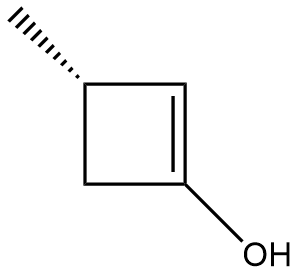
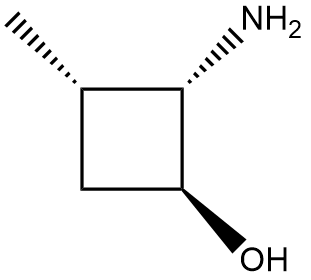
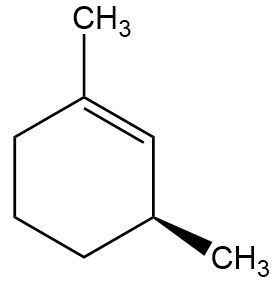
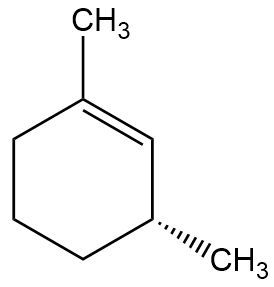
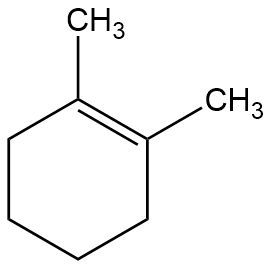
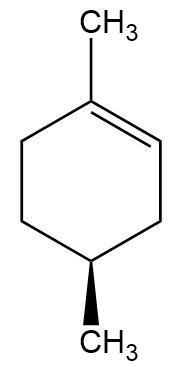
In the context of organic chemistry, the E2 reaction mechanism is a crucial pathway for the elimination of hydrogen halides from alkyl halides, leading to the formation of alkenes. This bimolecular elimination process involves the simultaneous removal of a proton (H+) and a leaving group (typically a halide, X-) from adjacent carbon atoms, resulting in the formation of a double bond.
To illustrate the E2 mechanism, consider a substrate with a good leaving group and a β-hydrogen. The reaction proceeds through a concerted mechanism, where the base abstracts the β-hydrogen while the leaving group departs, forming a double bond between the α and β carbons. The general reaction can be represented as:
R-CH2-CH(X)-B → R-CH=CH + HX + B-
In this equation, R represents the alkyl group, X is the leaving group, and B is the base. The product is an alkene (R-CH=CH) along with the byproducts HX (the hydrogen halide) and the conjugate base of the base used.
When drawing the final products of E2 reactions, it is essential to consider the stereochemistry of the substrate. The E2 mechanism typically favors an anti-coplanar arrangement of the leaving group and the hydrogen being removed, which can influence the stereochemical outcome of the product. This means that the most stable and favorable transition state occurs when the leaving group and the hydrogen are positioned 180 degrees apart.
In cases where the substrate has been modified to allow for a reaction where previously there was none, it is important to identify the new β-hydrogens available for elimination. This adjustment can lead to the formation of different alkenes, depending on the structure of the modified substrate.
Overall, understanding the E2 mechanism and its requirements is vital for predicting the products of elimination reactions in organic synthesis. By practicing with various substrates, students can enhance their ability to visualize and draw the mechanisms and products of these important reactions.