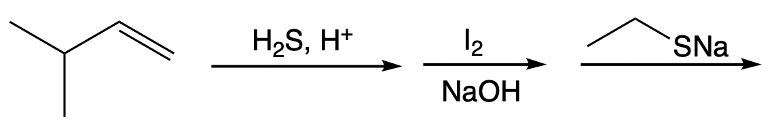
Disulfide bond formation is a crucial biochemical process involving the oxidation of thiols into disulfides, typically facilitated by mild oxidizing agents such as bromine (Br2) or iodine (I2) in a sodium hydroxide solution. A disulfide bond consists of two sulfur atoms covalently bonded together, and the formation of this bond occurs through a three-step mechanism.
In the first step, known as thiol ionization, a strong base, such as hydroxide ion (OH-), deprotonates the thiol group (R-SH), resulting in the formation of a thiolate anion (R-S-). This process involves the breaking of the bond between the sulfur and hydrogen, allowing sulfur to retain the electrons.
The second step involves an SN2 reaction where the thiolate anion reacts with a dihalide molecule. In this reaction, the sulfur atom uses its lone pairs to attack one of the halogen atoms (e.g., bromine), leading to the formation of a halogenated thiol (R-S-Br) as the bromine leaves as a good leaving group.
In the final step, another thiolate anion undergoes a second SN2 reaction with the halogenated thiol, resulting in the formation of the disulfide bond (R-S-S-R). Here, the sulfur from the thiolate anion attacks the sulfur of the halogenated thiol, displacing the halogen and completing the disulfide formation.
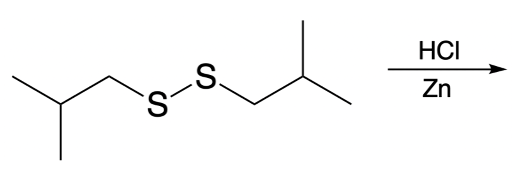
Importantly, disulfides can be reverted back to their original thiols through a process known as thiol reversion. This reduction is typically achieved using hydrochloric acid (HCl) in the presence of a zinc solution, which effectively breaks the disulfide bond and regenerates the two thiol molecules.
Understanding the mechanism of disulfide bond formation and reversion is essential in biochemistry, as these bonds play a significant role in the stability and function of proteins.