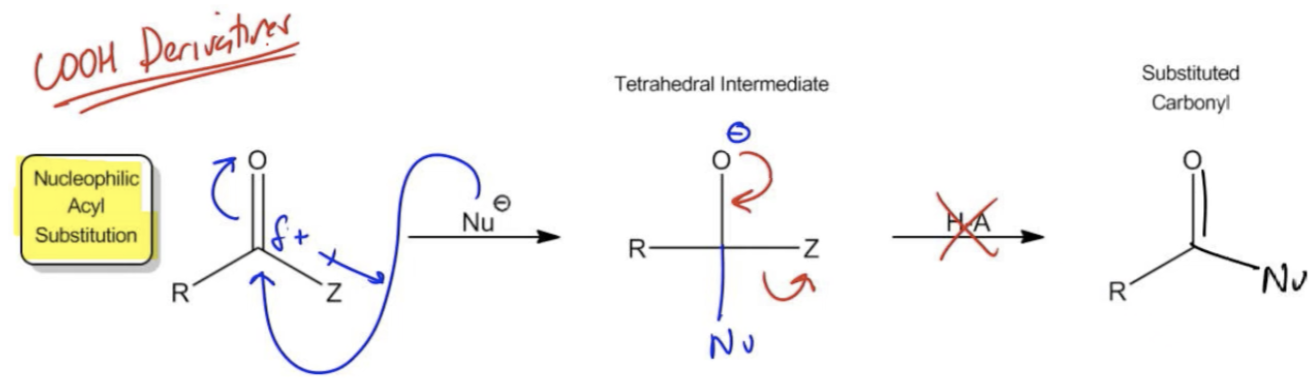
Carboxylic acid derivatives are a crucial category of organic molecules characterized by a carbonyl group (C=O) bonded to an electronegative substituent, referred to as a Z group, in the alpha position. Unlike ketones and aldehydes, which are limited to R (alkyl) or H (hydrogen) groups that are poor leaving groups, carboxylic acid derivatives feature Z groups that are more electronegative and thus better leaving groups. Common Z groups include chlorine, esters (OROH), and amines (NH2-), among others. These groups enhance the reactivity of the carbonyl, allowing for different chemical behavior.
One of the key mechanisms associated with carboxylic acid derivatives is nucleophilic acyl substitution (NAS). This mechanism differs significantly from the nucleophilic addition seen in ketones and aldehydes. In NAS, a nucleophile attacks the carbonyl carbon, leading to the substitution of the Z group. This process is reversible, and carboxylic acid derivatives can be hydrolyzed back to carboxylic acids using water in the presence of an acid or base. This hydrolysis is a defining feature of carboxylic acid derivatives, as it allows for the conversion back to the parent carboxylic acid.
Interestingly, nitriles also qualify as carboxylic acid derivatives due to their ability to undergo hydrolysis to form carboxylic acids. This highlights the versatility of carboxylic acid derivatives in organic chemistry, as they can be transformed into carboxylic acids through specific reactions.
Understanding these concepts is essential for grasping the differences in reactivity and mechanisms between various classes of organic compounds, particularly when comparing the behavior of carboxylic acid derivatives to that of ketones and aldehydes.