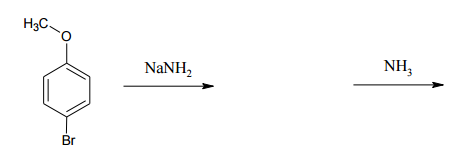Benzene can undergo various substitution mechanisms, including electrophilic and nucleophilic pathways. One intriguing method is the benzyne pathway, which involves an elimination followed by an addition process. This pathway produces an unstable intermediate known as an aryne or benzyne, characterized by a triple bond within the aromatic ring. The presence of a triple bond creates significant strain, as the ideal bond angle for a triple bond is 180 degrees, while the benzene ring forces it into a 120-degree angle, leading to instability.
In contrast to the electrophilic aromatic substitution (EAS) and nucleophilic aromatic substitution (SNAr) mechanisms, the benzyne pathway begins with an elimination step. This elimination is typically achieved through an E2 mechanism, where a strong base abstracts a hydrogen atom from the benzene ring, resulting in the formation of the benzyne intermediate. The reaction can be represented as follows:
For the E2 elimination:
\[\text{C}_6\text{H}_5\text{X} + \text{Base} \rightarrow \text{C}_6\text{H}_4^{\text{(triple bond)}} + \text{HX}\]
Once the unstable benzyne is formed, the next step involves the addition of a nucleophile. A common nucleophile used in this pathway is the conjugate acid of the base that initiated the elimination. The nucleophile attacks one side of the triple bond, resulting in the formation of a new intermediate. This can be illustrated as:
For the nucleophilic addition:
\[\text{C}_6\text{H}_4^{\text{(triple bond)}} + \text{B}^+ \rightarrow \text{C}_6\text{H}_4\text{B} + \text{B}^-\]
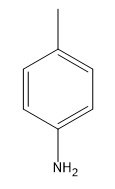
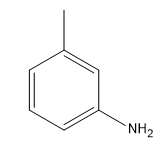
After the addition, a proton exchange occurs, allowing the final product to be formed. This pathway can be utilized to synthesize aniline from an aryl halide and a strong base such as \(\text{NH}_2^-\). The overall reaction can be summarized as:
Starting with an aryl halide:
\[\text{C}_6\text{H}_5\text{X} + \text{NH}_2^- \rightarrow \text{C}_6\text{H}_5\text{NH}_2 + \text{HX}\]
This method highlights the unique nature of the benzyne pathway, showcasing how an unstable intermediate can lead to the formation of valuable compounds like aniline through a series of elimination and addition reactions.