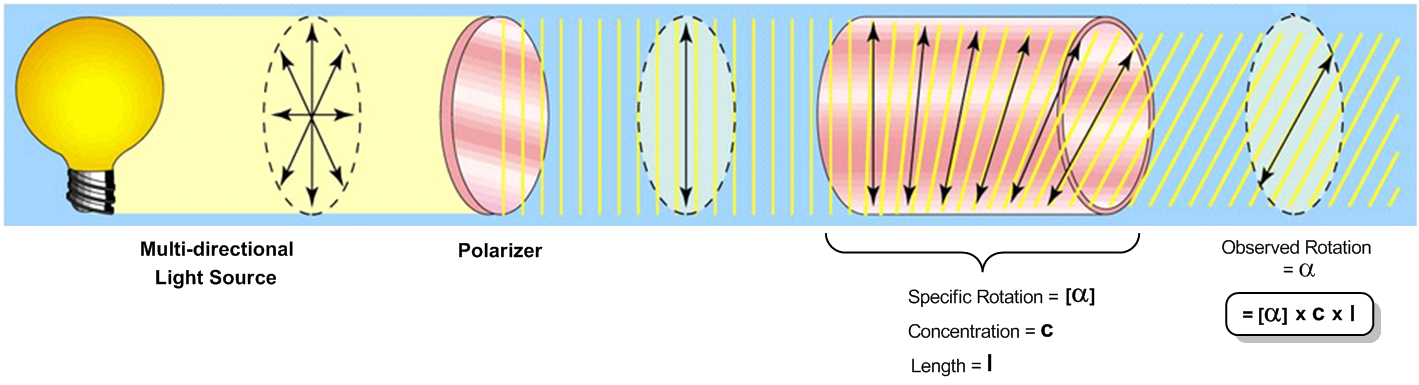
Optical activity is a unique property of chiral molecules, which can rotate plane polarized light when it passes through them. This phenomenon is measured using a device called a polarimeter. The process begins with a light source, typically a bulb that emits multi-directional light. This light first passes through a polarizer, which filters it to create plane polarized light, allowing it to travel in a single direction.
Next, the plane polarized light enters a tube containing a chiral mixture. As the light travels through this chiral substance, it experiences a rotation in its plane, which is a characteristic feature of chiral molecules. The extent of this rotation is influenced by several factors: the specific rotation of the molecule, the concentration of the chiral substance, and the length of the tube through which the light passes. The specific rotation is a unique value for each chiral compound, representing the maximum rotation that can occur with a pure enantiomer. It is important to note that specific rotation is not predictable based on molecular size or chirality.
The relationship between these variables can be expressed with the formula for observed rotation:
\[ \text{Observed Rotation} = \text{Specific Rotation} \times \text{Concentration} \times \text{Length} \]
This means that increasing the concentration or the length of the tube will result in a greater observed rotation. Conversely, if the observed rotation, concentration, and length are known, one can rearrange the formula to solve for specific rotation:
\[ \text{Specific Rotation} = \frac{\text{Observed Rotation}}{\text{Concentration} \times \text{Length}} \]
Rotations can be classified as either dextrorotary or levorotary. A clockwise rotation is termed dextrorotary and is denoted with a positive sign, while a counterclockwise rotation is called levorotary and is indicated with a negative sign. It is crucial to understand that these designations do not correlate with the chirality of the molecule itself. For instance, a molecule with an R configuration may exhibit either a positive or negative rotation, depending on its specific properties. Thus, the terms dextrorotary and levorotary simply describe the direction of light rotation and do not provide information about the chirality of the molecule.