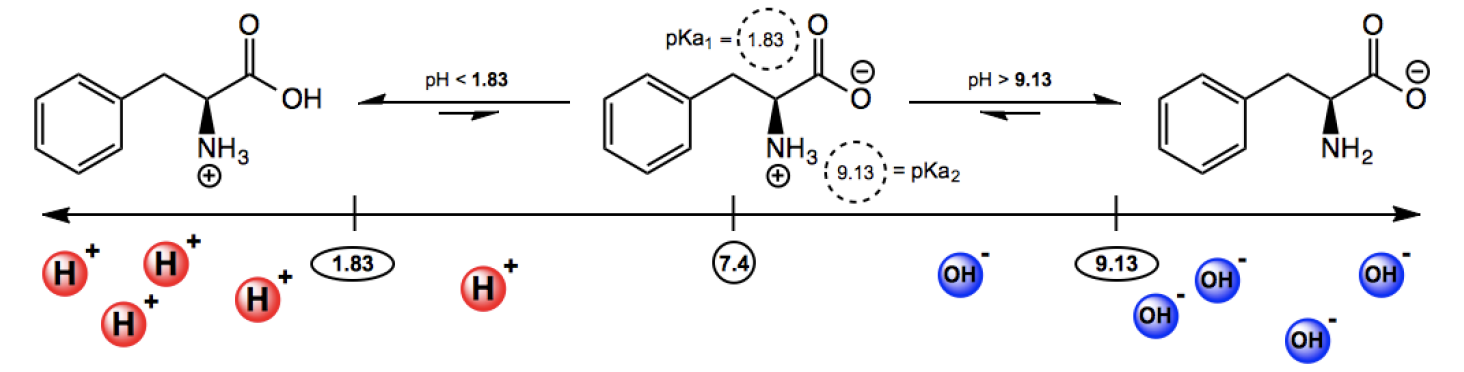
Amino acids, at physiological pH (approximately 7.4), do not exist in their neutral forms as previously represented. Instead, they typically exist as zwitterions, which are molecules that have both positive and negative charges but maintain a net charge of zero. This state arises due to the equilibrium between the carboxylic acid and amine groups within the amino acid structure.
At physiological pH, the carboxylic acid group loses a hydrogen ion (H+), becoming negatively charged (anion), while the amine group gains a hydrogen ion, resulting in a positively charged ammonium group (cation). This shift can seem counterintuitive since neutral structures are often considered more stable. However, the stability of zwitterions can be explained through acid-base chemistry principles, particularly the concept of pKa.
The pKa is a measure of the strength of an acid; the lower the pKa, the stronger the acid. For amino acids, the pKa of the carboxylic acid group is around 2, while the pKa of the ammonium group is approximately 9. When considering the movement of the hydrogen ion, it is more favorable for the hydrogen to associate with the ammonium group due to its higher pKa, indicating it is the weaker acid. Thus, the equilibrium favors the zwitterion form, where the hydrogen ion shifts from the carboxylic acid to the amine group.
Understanding the zwitterionic form of amino acids is crucial, as the pH of the environment can influence the charge states of these molecules. Variations in pH will lead to different predominant forms of amino acids, which will be explored further in subsequent discussions.