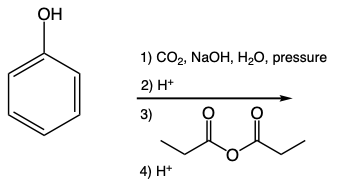
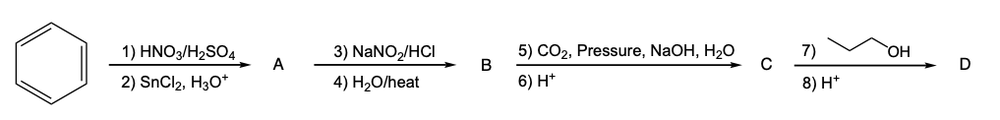
The Colby-Schmidt reaction is a specific type of electrophilic aromatic substitution (EAS) that synthesizes ortho-hydroxybenzoic acid, commonly known as salicylic acid. This reaction begins with phenol, which, under basic conditions, is deprotonated to form phenoxide. The phenoxide ion then acts as a nucleophile, allowing for the carboxylation at the ortho position of the benzene ring.
In this process, carbon dioxide is introduced in the presence of sodium hydroxide and water, followed by the application of high pressure. This combination facilitates the attachment of carbon dioxide to the benzene ring, ultimately leading to the formation of a carboxylic acid after protonation. A crucial aspect of the Colby-Schmidt reaction is the preference for the ortho position for substitution, despite the general tendency for substituents to favor the para position to minimize steric hindrance.
The ortho preference in this reaction can be attributed to intramolecular hydrogen bonding. The oxygen atom of the carbonyl group can form a hydrogen bond with the hydroxyl group of the phenol, enhancing the stability of the resulting molecule. This intramolecular interaction makes the ortho product more favorable in this context, demonstrating that the presence of intramolecular hydrogen bonding can influence the regioselectivity of electrophilic aromatic substitutions.
In summary, the Colby-Schmidt reaction effectively transforms phenol into salicylic acid by utilizing the unique properties of phenoxide and the stabilizing effects of intramolecular hydrogen bonding, highlighting the importance of molecular interactions in determining reaction pathways.