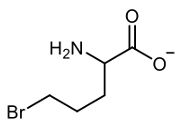
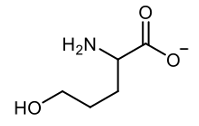
Amino acid synthesis can be effectively achieved through the acetoamido malonic ester synthesis, which is a variation of the malonic ester synthesis. This process involves the use of diethyl acetamido malonic ester, which consists of diethyl groups, an acetamido group, and a malonic acid derivative. The synthesis occurs in three main steps, with the final step divided into two sub-steps for clarity.
The first step is enolization, where a strong base, typically ethoxide, deprotonates the alpha carbon of the acetoamido malonic ester. This reaction results in the formation of an enolate ion, characterized by a negatively charged carbon that holds onto a lone pair of electrons. The enolate serves as a reactive intermediate for the subsequent steps.
In the second step, the enolate undergoes alkylation. Here, the enolate anion attacks an alkyl halide through an SN2 mechanism, resulting in the attachment of a methyl group to the alpha carbon. This step transforms the enolate into an alkylated acetoamido malonic ester.
The third step is divided into two parts: hydrolysis and decarboxylation. In step 3a, hydrolysis occurs, where the amide and ester groups are converted back into their respective carboxylic acids. This process involves cleaving the amide bond, resulting in a protonated amino group and a carboxylic acid. The focus here is on the formation of alkyl aminomalonic acid.
Step 3b involves decarboxylation, where one of the carboxylic acid groups is eliminated as carbon dioxide upon the application of heat. This final transformation yields the desired amino acid product. Overall, the acetoamido malonic ester synthesis is a multi-step process that builds upon the foundational principles of malonic ester synthesis, ultimately leading to the formation of amino acids through a series of well-defined chemical reactions.