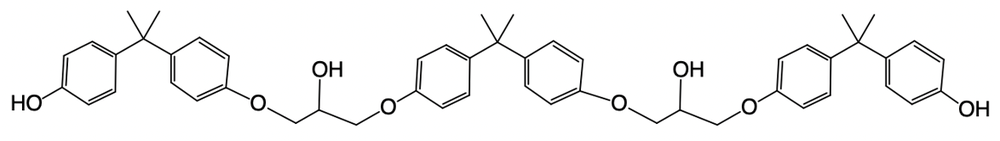
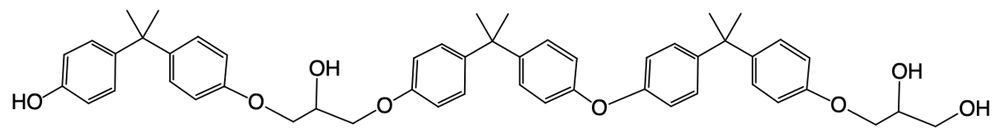
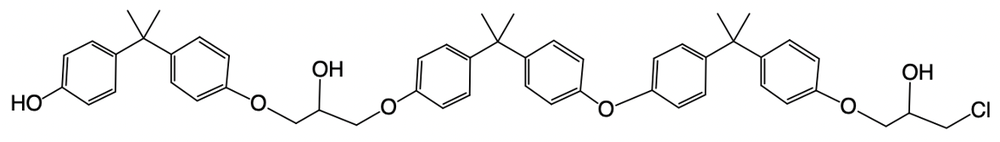
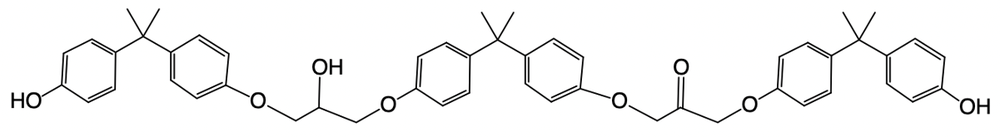
Epoxy resins are formed through a complex mechanism that can vary in the number of steps involved. The general formula for determining the number of steps in the formation process is given by:
Number of steps = 2 × (number of moles of BPA) + 1 (protonation step).
In this context, BPA, or bisphenol A, is a crucial molecule in the synthesis of epoxy resins. Another important compound to understand is epichlorohydrin, which consists of an epoxide ring attached to a methylene carbon (–CH2–) and a chlorine atom (–Cl). This structure plays a significant role in the reaction mechanism leading to the formation of epoxy resins.
Understanding these components and their interactions is essential for grasping the overall process of epoxy resin formation. As you delve deeper into the mechanism, you'll see how these molecules contribute to the final product.