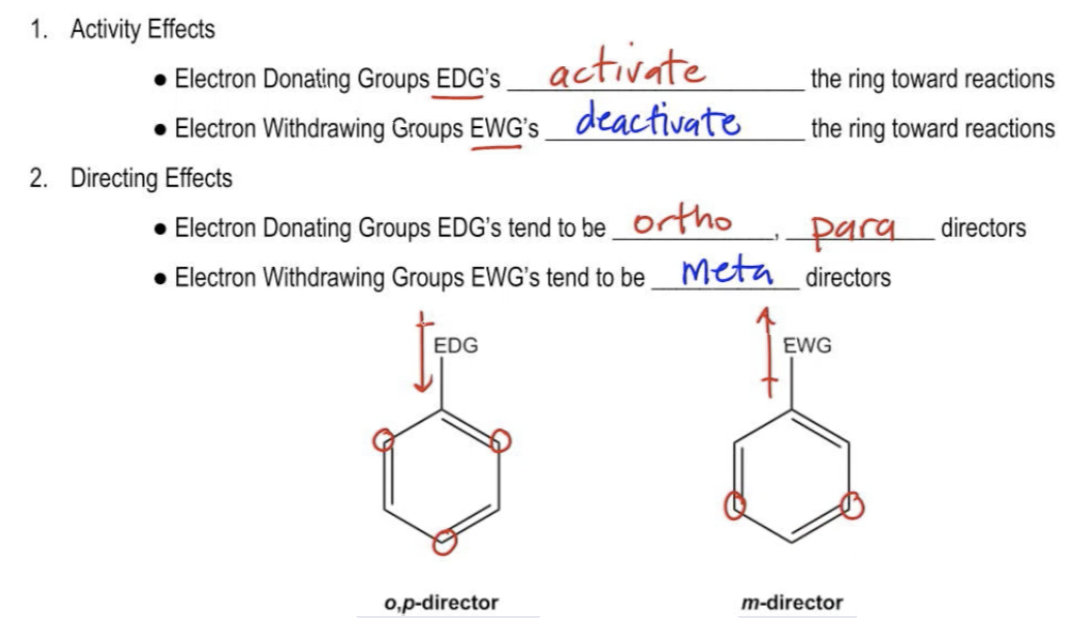
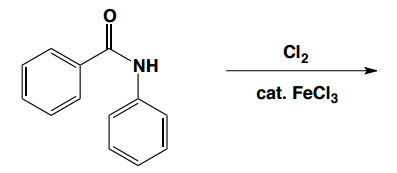
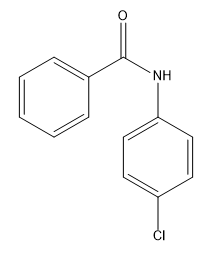
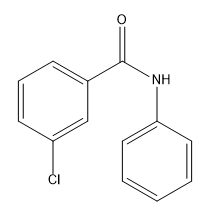
In the study of electrophilic aromatic substitution (EAS), understanding how substituents on a benzene ring influence subsequent reactions is crucial. When a substituent is already present on the benzene, it can significantly alter the electron density of the ring, affecting both its reactivity and the position where additional substituents will add. This leads us to the concepts of electron donating groups (EDGs) and electron withdrawing groups (EWGs), which are fundamental in organic chemistry.
Electron donating groups increase the electron density of the benzene ring, making it more nucleophilic and thus more reactive towards further EAS reactions. This means that if the first substituent is an EDG, the benzene will be activated for subsequent reactions, allowing for easier addition of a second substituent. Common examples of EDGs include alkyl groups and -OH (hydroxyl) groups, which direct new substituents to the ortho and para positions relative to themselves, hence they are referred to as ortho/para directors.
Conversely, if the first substituent is an electron withdrawing group, it decreases the electron density of the benzene ring, making it less nucleophilic and less reactive towards further EAS reactions. This deactivation means that the second reaction will be more challenging than the first, and the benzene will be less reactive than unmodified benzene. EWGs, such as nitro groups (-NO2) and carbonyl groups (like -C=O), direct subsequent reactions to the meta position, classifying them as meta directors.
To summarize, the nature of the first substituent on a benzene ring plays a pivotal role in determining both the reactivity of the ring and the position of any additional substituents. Recognizing whether a group is an electron donating or withdrawing group is essential for predicting the outcomes of EAS reactions. Understanding these concepts will not only aid in mastering organic chemistry but will also be invaluable in future scientific studies and applications.