In organic chemistry, substitution reactions can occur through two primary mechanisms: SN2 and SN1. While the SN2 mechanism involves a strong, negatively charged nucleophile attacking an accessible leaving group in a single step, the SN1 mechanism operates quite differently. In SN1 reactions, a neutral nucleophile interacts with an inaccessible leaving group, leading to substitution through a two-step process.
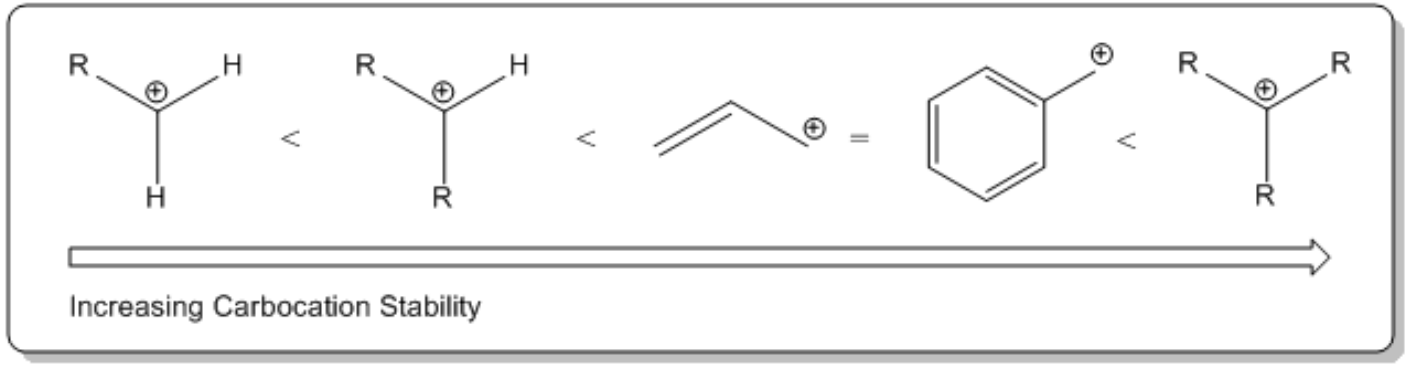
The first step of the SN1 mechanism is characterized by the departure of the leaving group, which occurs spontaneously without the nucleophile's involvement. This is a significant departure from the SN2 mechanism, where bond formation precedes bond breaking. In SN1, the leaving group, often a halogen, dissociates to form a carbocation and a negatively charged ion. The stability of the carbocation is crucial; it is typically sp2 hybridized and exhibits a trigonal planar geometry, allowing for potential attack from either side by the nucleophile.
The dissociation constant for alkyl halides is generally higher than that of water, indicating that alkyl halides can ionize more readily. This is due to the weak carbon-halogen bond and the strong dipole present in alkyl halides, which facilitates the formation of the carbocation intermediate. The carbocation, although unstable, can be isolated and serves as a strong electrophile in the subsequent step.
In the second step, the neutral nucleophile, which was previously inactive, now attacks the carbocation. Unlike in SN2 reactions, where the nucleophile must approach from the back side, the planar structure of the carbocation allows the nucleophile to attack from either the front or the back. This results in the formation of two possible products, leading to the generation of enantiomers if the carbon center is chiral. The outcome is a racemic mixture, as there is an equal probability of the nucleophile attacking from either side.
Ultimately, both SN1 and SN2 mechanisms yield substitution products, but they differ significantly in their pathways, the nature of the nucleophile, and the stereochemical outcomes. Understanding these differences is essential for predicting the behavior of various organic compounds during substitution reactions.