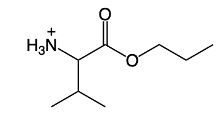
Amino acids can undergo various chemical reactions, one of which is esterification. This process, specifically Fischer esterification, involves the reaction of a carboxylic acid group with an alcohol to form an ester. In the context of amino acids, the carboxylic acid group can be transformed into an ester group by reacting with an alcohol, resulting in the formation of an ester, represented as R-O-R'. Here, R denotes the alkyl group from the alcohol, while R' represents the rest of the amino acid structure.
Conversely, if we wish to revert the ester back to the original carboxylic acid, we can employ hydrolysis using strong acids such as hydrochloric acid (HCl) or sulfuric acid (H₂SO₄). This hydrolysis process effectively breaks the ester bond, regenerating the carboxylic acid group. The mechanism for this transformation follows the nucleophilic acyl substitution pathway, which is consistent with the reactions of carboxylic acid derivatives. For a deeper understanding of the mechanism, it is beneficial to review the relevant sections on carboxylic acid derivatives.
In summary, the ability of amino acids to undergo esterification highlights their versatility in organic reactions, allowing for the conversion of carboxylic acid groups into esters and vice versa through hydrolysis.