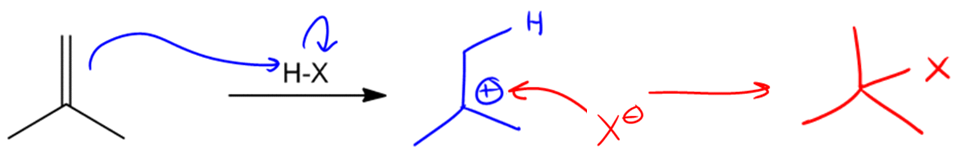
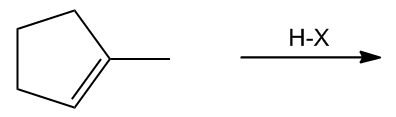In the study of alkenes, particularly those with asymmetric double bonds, understanding how to predict the formation of products during electrophilic addition reactions is crucial. This is where Markovnikov's Rule comes into play. This rule is essential for determining the outcome of reactions involving alkenes that have different substituents on either side of the double bond.
Markovnikov's Rule states that when an asymmetric alkene reacts with an electrophile, the more stable carbocation intermediate will form. This means that the hydrogen atom (or electrophile) will attach to the carbon atom that can best stabilize the positive charge of the carbocation. In simpler terms, the rule can be summarized as: the electrophile adds to the carbon with the greater number of hydrogen atoms, leading to the formation of the more stable carbocation.
To illustrate this, consider an asymmetric double bond where the addition of an electrophile can lead to two possible products. If we denote the two carbons of the double bond as A and B, with A having more hydrogen atoms than B, the hydrogen will preferentially attach to carbon A. Consequently, the carbocation will form on carbon B, which is less stable if it is a primary carbocation, compared to a tertiary carbocation that could form on carbon A.
Carbocations are classified based on their stability: tertiary carbocations are more stable than secondary, which are more stable than primary. This stability is influenced by the number of alkyl groups attached to the positively charged carbon. Therefore, in a reaction where a tertiary carbocation can form, it will be favored over a primary carbocation, leading to a single product that aligns with Markovnikov's Rule.
As a result, when performing reactions with asymmetric alkenes, one can confidently predict that the product formed will be the one corresponding to the more stable carbocation, ensuring that the reaction yields a single, predominant product rather than a mixture. This understanding is fundamental for predicting the outcomes of various organic reactions involving alkenes.