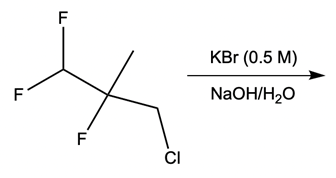
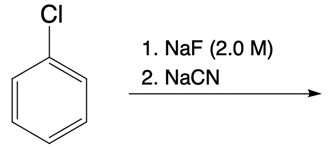
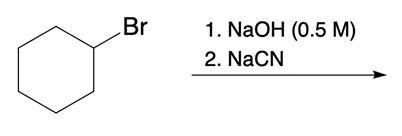
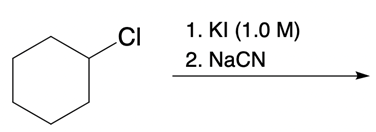
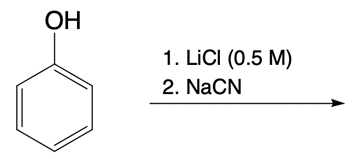
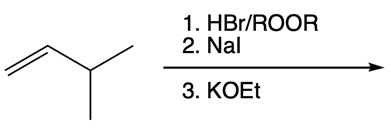
Nucleophilic catalysis is a fascinating concept in organic chemistry, particularly in the context of substitution reactions. In the context of the SN2 mechanism, we observe that a nucleophilic catalyst can enhance the reaction by replacing a leaving group with a more effective nucleophile or leaving group. This process is particularly relevant when considering the trends in the periodic table, especially among the halogens in Group 7A. As we move down this group, the atomic size increases, which influences both nucleophilicity and leaving group ability. For instance, iodine, being larger than bromine, chlorine, and fluorine, serves as a better nucleophile and leaving group compared to its lighter counterparts.
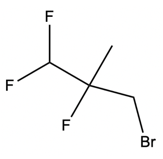
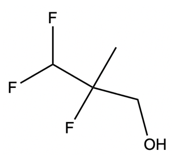
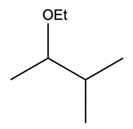
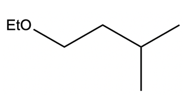
In a typical SN2 reaction involving a chiral center, the mechanism begins with an alkyl halide where the halogen is displaced by a larger halogen, resulting in an inversion of configuration. This inversion occurs because the nucleophile attacks from the opposite side of the leaving group, leading to a change from a wedged to a dashed bond representation. In the case of double SN2 reactions, this process occurs twice. After the first inversion, a second nucleophile can attack, displacing the first halogen and causing another inversion. However, since the second halogen is a better leaving group, the overall configuration can return to its original state, resulting in retention of the stereochemistry.
This dual inversion mechanism not only illustrates the concept of retention but also highlights the efficiency of nucleophilic catalysis. By replacing the initial halogen with a more effective nucleophile, the rate of the subsequent SN2 reaction is significantly increased. Understanding these dynamics is crucial for predicting reaction outcomes and optimizing conditions in synthetic organic chemistry.