In organic chemistry, a condensation reaction occurs when two molecules combine to form a larger molecule, accompanied by the loss of a smaller molecule, often water. A key player in many condensation reactions is the enolate, which is formed when an alpha proton is removed from a carbonyl compound, resulting in a negatively charged anion at the alpha carbon. This reactivity allows enolates not only to react with electrophiles but also to engage in self-condensation, where they react with themselves in the absence of an external electrophile.
To understand this process, it's essential to recall the concept of nucleophilic addition, where nucleophiles attack the electrophilic carbon of carbonyl compounds, leading to the formation of tetrahedral intermediates. In the case of enolates, when a base deprotonates an alpha carbon, the resulting enolate can act as a nucleophile. If no electrophile is present, the enolate can react with the non-enolated version of itself, resulting in a nucleophilic addition that forms a new tetrahedral intermediate. This intermediate ultimately leads to the formation of a larger molecule through a condensation reaction.
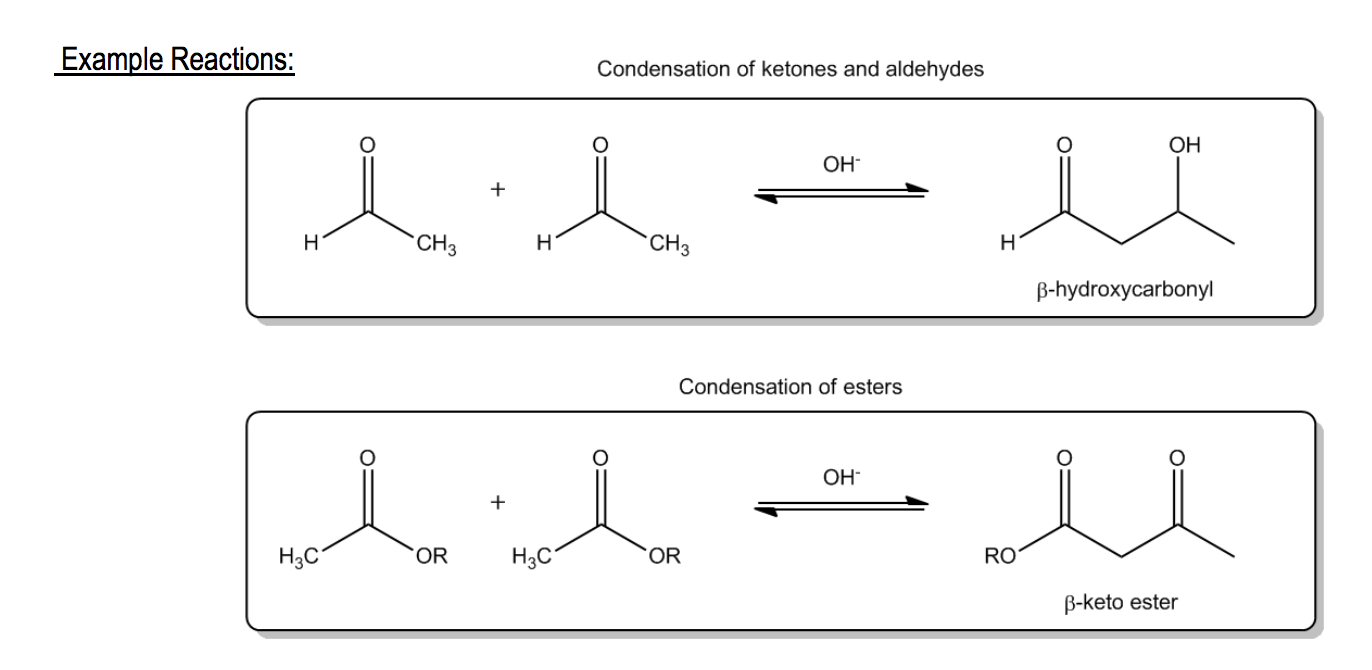
For example, when two ketones or aldehydes undergo this process, they can form a compound that contains both alcohol and carbonyl functional groups, resulting in what is known as an aldol reaction. This reaction exemplifies condensation as it combines two smaller molecules into one larger structure. Similarly, when two esters react through enolate self-condensation, they can yield a beta-dicarbonyl compound, specifically a beta-ketoester, in a reaction termed Claisen condensation. These reactions highlight the versatility of enolates in forming complex organic molecules through condensation mechanisms.
Overall, the study of enolate-mediated condensation reactions, including aldol and Claisen condensations, will be a focal point in understanding how smaller organic molecules can combine to create larger, more complex structures.