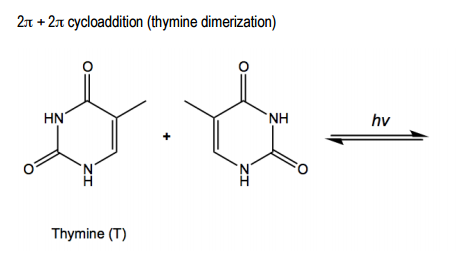
Photochemical cycloaddition is a unique type of pericyclic reaction that occurs when two pi bonds are destroyed through a light-activated mechanism, rather than a heat-activated one. This process results in the formation of a cyclobutane from two alkenes, effectively eliminating the two double bonds present in the reactants. The reaction is characterized by a cyclic and concerted mechanism, where the double bonds simultaneously form new single bonds, leading to the final product without a clear starting or ending point.
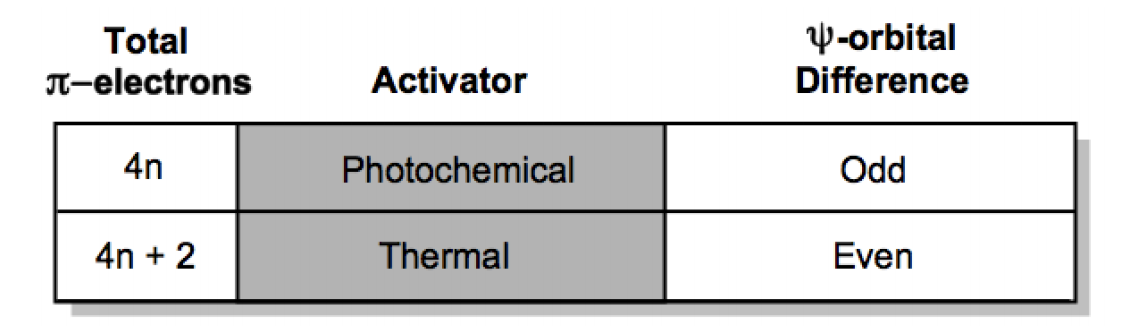
To determine whether the product of a photochemical cycloaddition is favored, one can utilize frontier molecular orbital (FMO) theory. In any cycloaddition, the highest occupied molecular orbital (HOMO) of one reactant must interact with the lowest unoccupied molecular orbital (LUMO) of another. The effectiveness of this interaction is enhanced when the orbital symmetry and energy levels of the HOMO and LUMO align closely. In photochemical reactions, light excites electrons in a conjugated system, allowing them to transition from a bonding orbital (Psi 1) to an antibonding orbital (Psi 2), thus altering the HOMO and LUMO characteristics of the reactants.
For example, when light interacts with alkene A, an electron from its HOMO is promoted to a higher energy state, changing the identity of the orbitals involved in the reaction. This shift can create a situation where the HOMO of alkene A and the LUMO of alkene B are nearly at the same energy level, resulting in a minimal HOMO-LUMO gap. This close energy alignment indicates a favorable reaction pathway, as the symmetry of the orbitals allows for effective overlap, making the process symmetry allowed. In contrast, thermal cycloadditions typically involve symmetry disallowed interactions, which do not lead to a reaction.
In summary, photochemical cycloaddition is a fascinating reaction that leverages light to facilitate the formation of new bonds while altering the electronic structure of the reactants. Understanding the principles of FMO theory and the role of light in exciting electrons is crucial for predicting the favorability of these reactions.