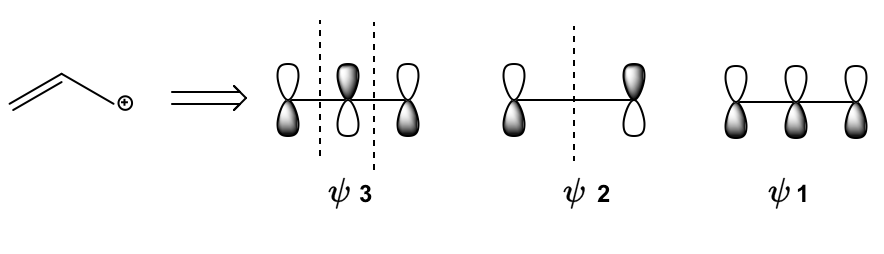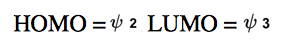In understanding molecular orbital diagrams for three-atom conjugated systems, it is essential to recognize the significance of the allylic position. The allylic position refers to the site adjacent to a double bond, which plays a crucial role in resonance. In a three-atom system, the presence of a double bond typically leads to an allylic position that can resonate, allowing electrons to shift between different atoms. This resonance can be effectively explained through molecular orbital theory.
When analyzing a propanyl ion, for instance, we consider three atomic orbitals, labeled A, B, and C. The double bond contributes two pi electrons, which occupy the most stable bonding molecular orbital, denoted as ψ1. Any additional electrons, whether they are from an empty orbital, a radical, or a lone pair, will occupy the next available molecular orbital, ψ2. Notably, ψ2 contains a node at atom B, indicating that no electrons can pass through this atom. Consequently, this means that the allylic position cannot react at atom B, but can only react at atoms A or C.
This molecular orbital framework provides a deeper understanding of why resonance structures behave as they do. For example, while an allylic ion can resonate to the other side of the double bond, it cannot transition to the middle atom (B) because there are no electrons present in that orbital. Thus, the molecular orbital theory not only clarifies the behavior of allylic positions but also reinforces the principles of resonance that have been previously established.