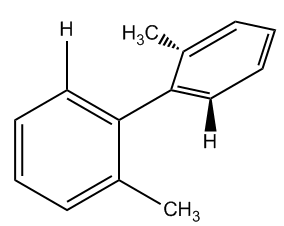
In the study of stereochemistry, it's essential to understand that chirality is typically associated with the presence of chiral centers, which are carbon atoms bonded to four different substituents. However, there are exceptions to this rule, notably a class of compounds known as atropoisomers. These unique compounds exhibit chirality despite lacking any chiral centers due to their restricted rotation.
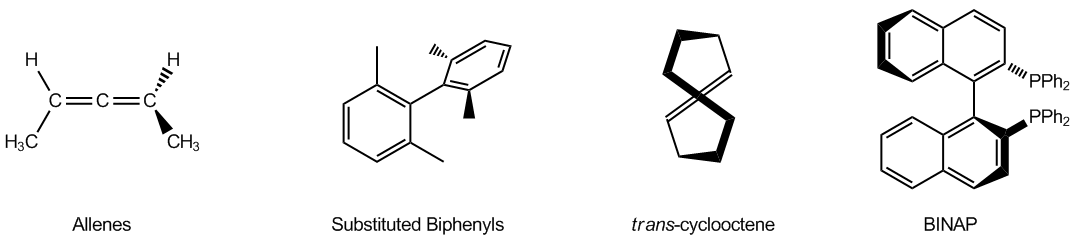
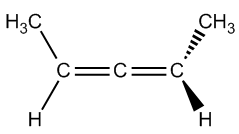
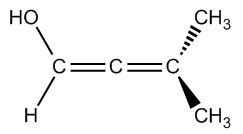
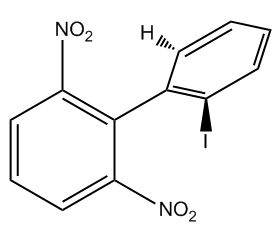
Atropoisomers arise from structural features that prevent free rotation around certain bonds. For instance, allenes are compounds with two adjacent double bonds that cannot rotate, leading to chirality. Similarly, substituted biphenyls consist of two phenyl groups connected in a way that their substituents hinder rotation, effectively locking the structure in place. Another example is trans cyclooctene, which can exist in right-handed and left-handed forms due to its inability to rotate, despite being a twisted structure.
Additionally, BINAP (which stands for 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl) is another example of a compound that displays chirality without chiral centers. In BINAP, one of the phenyl groups acts as a barrier to rotation across a single bond, contributing to its chiral nature.
These examples illustrate that chirality can manifest in various ways beyond the conventional understanding of chiral centers. While atropoisomers are not commonly encountered in introductory organic chemistry courses, they serve as an important reminder of the complexity and diversity of molecular structures and their properties.