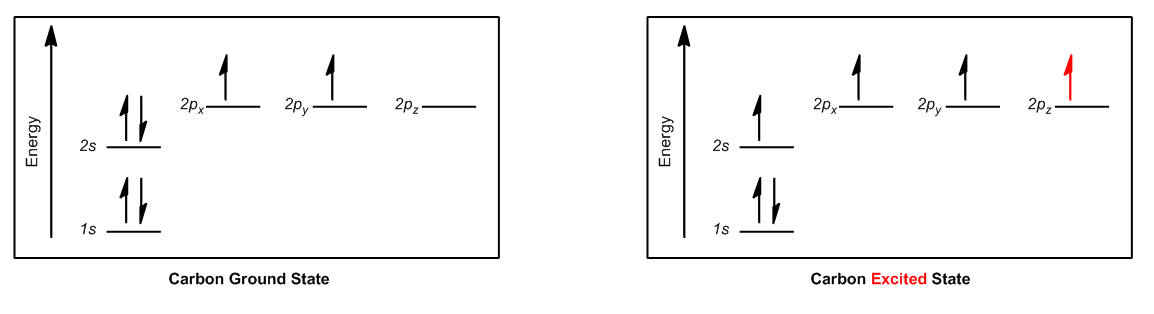
The Aftbaugh principle is fundamental in understanding electron configurations, stating that electrons fill orbitals in order of increasing energy. For instance, in the case of carbon, the electron configuration reveals that the 1s orbital is fully occupied, followed by the 2s orbital, which is also full. However, carbon has only two electrons in its three available 2p orbitals, leading to an incomplete filling of these orbitals.
When visualizing the orbital diagram for carbon, it becomes evident that while the 1s and 2s orbitals are filled, the 2p orbitals present a challenge. With two electrons distributed among three p orbitals, the configuration is not optimal for stability. This situation prompts the question of why carbon forms four bonds instead of just two, given its partially filled p orbitals.
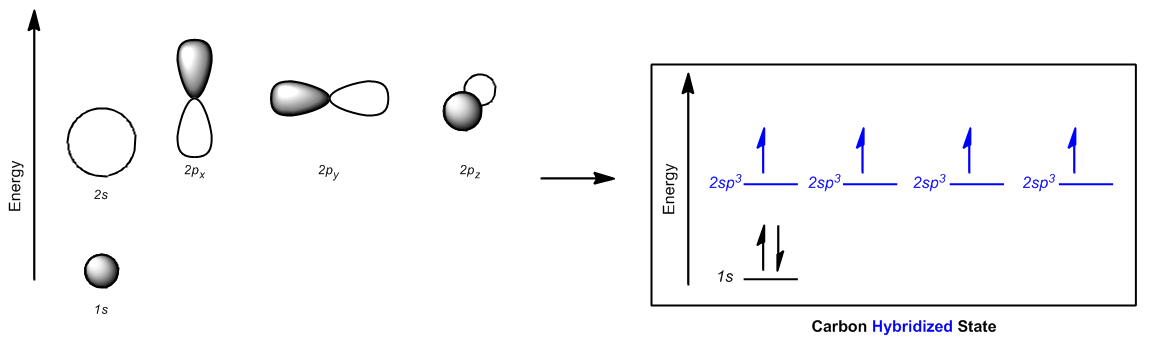
The answer lies in the concept of stability and bonding preferences. Carbon, in its quest for stability, can promote one of its 2s electrons to a higher energy 2p orbital. This process, although it appears to violate the Aftbaugh principle, allows carbon to achieve a configuration where all four orbitals (one 2s and three 2p) are now partially filled. This arrangement is more favorable because it enables carbon to form four equivalent bonds, leading to greater stability through covalent bonding.
In summary, while the initial electron configuration of carbon suggests a limited bonding capacity, the excitation of an electron into a higher energy state facilitates the formation of four bonds. This results in a more stable molecular structure, demonstrating the intricate balance between energy levels and chemical bonding in elements.