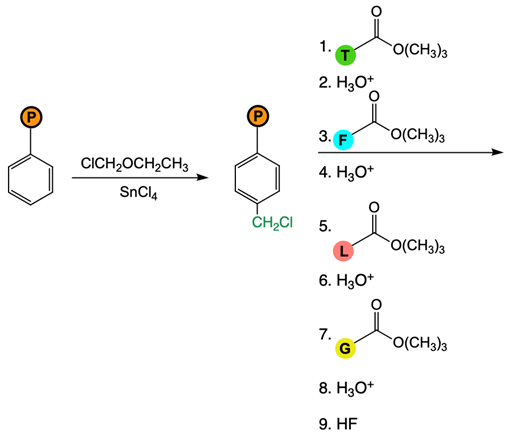
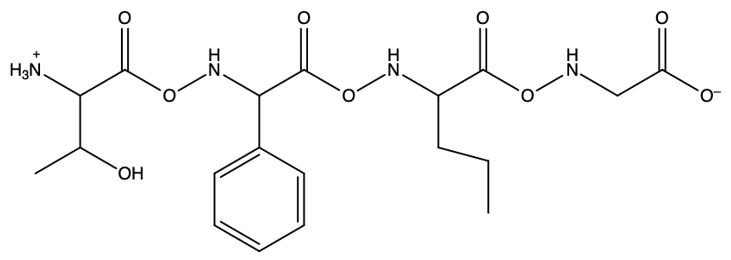
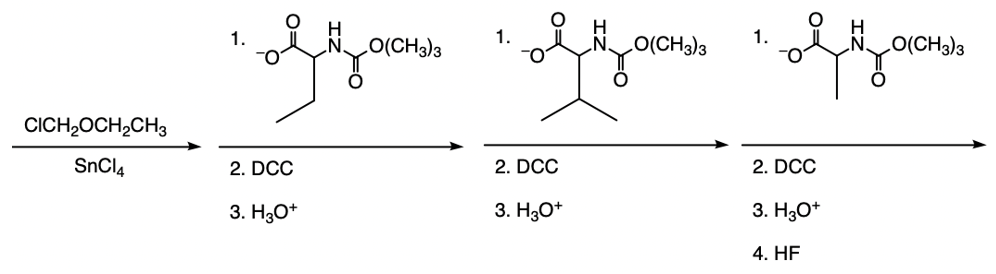
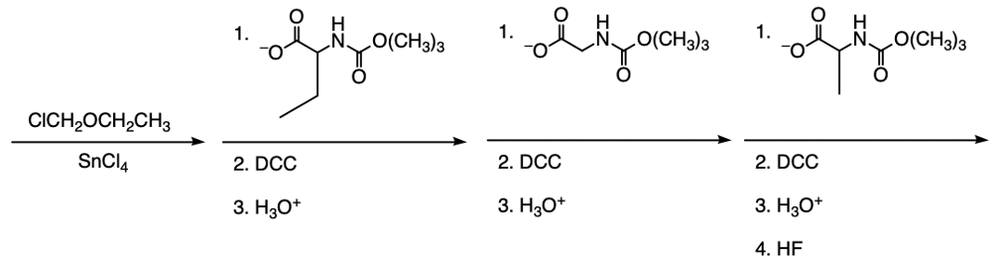
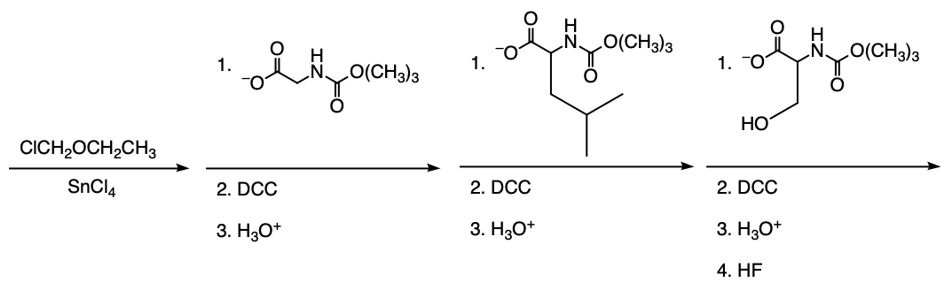
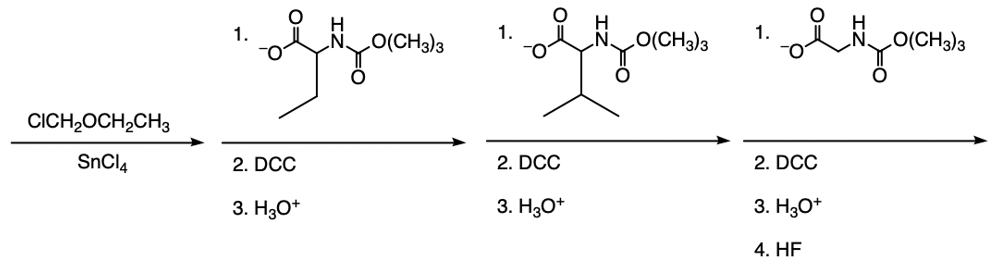
The Merrifield solid phase peptide synthesis (SPPS) is an automated technique used to construct peptide chains on a solid support, specifically utilizing polystyrene beads. Polystyrene is a polymer formed through the polymerization of styrene, which consists of a benzene ring bonded to two double-bonded carbons. In the context of Merrifield synthesis, chloromethylated polystyrene is employed, where a chloromethyl group (–CH2Cl) is introduced to the polystyrene structure.
To initiate the synthesis, a protected amino acid is used. Typically, amino acids have a free amino group, but in this process, the amino group is protected by an acetyl or acyl group. This protection is crucial as it prevents unwanted reactions during the synthesis. The reaction occurs through an SN2 mechanism, where the oxygen from the protected amino acid attacks the methylene carbon of the chloromethyl group, displacing the chlorine atom and forming a covalent bond between the amino acid and the polystyrene bead.
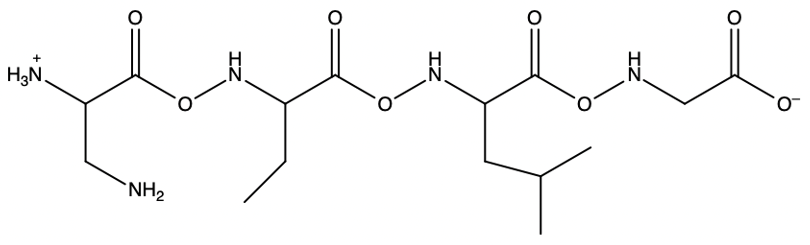
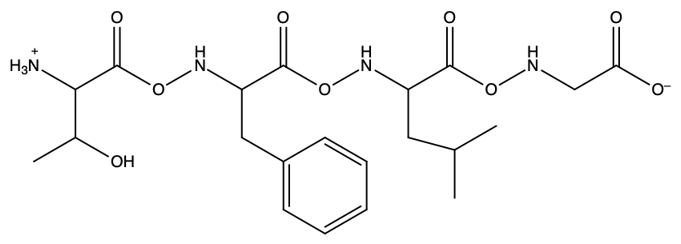
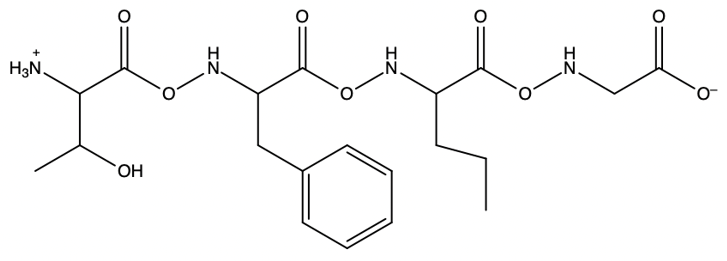
The process allows for the sequential addition of amino acids, effectively growing the peptide chain on the solid support. This method provides the flexibility to synthesize peptides of varying lengths. Once the desired peptide chain is formed, it can be cleaved from the polystyrene bead, allowing for the isolation of the synthesized peptide. Overall, the Merrifield solid phase peptide synthesis method is a powerful tool in peptide chemistry, enabling efficient and controlled peptide synthesis.