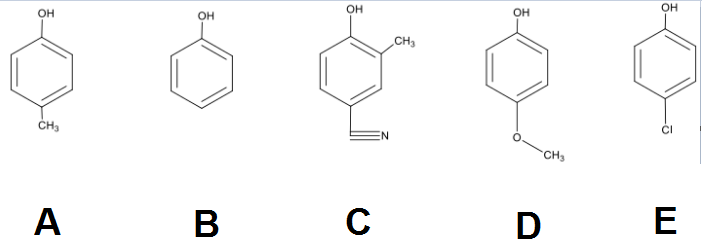
Phenols are a class of compounds that exhibit greater acidity compared to regular alcohols, primarily due to the resonance effect. This effect is crucial in understanding acidity, as it relates to the stability of the conjugate base formed after the acid donates a proton. When phenol loses a proton, it forms phenoxide, which carries a negative charge. This negative charge can be stabilized through resonance, allowing it to delocalize across the aromatic ring. As a result, the pKa of phenol is approximately 10, significantly lower than the pKa of typical alcohols, which is around 16. This lower pKa indicates that phenol is more willing to donate its proton, making it a stronger acid.
To further analyze the acidity of phenols, it is essential to consider the influence of substituents on the aromatic ring. Electron-donating groups (EDGs) and electron-withdrawing groups (EWGs) play pivotal roles in this context. EDGs, which push electron density into the ring, tend to decrease acidity. This is because they destabilize the already negatively charged conjugate base, making it less favorable for the acid to lose its proton. Conversely, EWGs, which pull electron density away from the ring, enhance acidity. By stabilizing the conjugate base through the removal of electron density, EWGs make it easier for the acid to donate its proton.
In summary, the acidity of phenols is influenced by the resonance stabilization of the phenoxide ion and the nature of substituents on the aromatic ring. Understanding these concepts allows for the prediction of acidity in various phenolic compounds, where the presence of electron-withdrawing groups generally leads to increased acidity, while electron-donating groups result in decreased acidity.