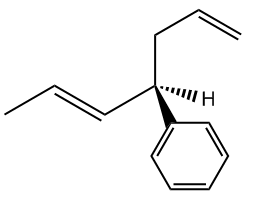
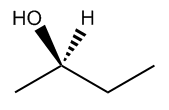
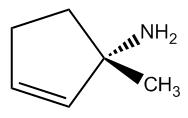
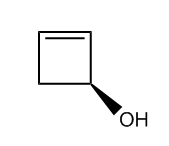
Understanding chiral centers is crucial in organic chemistry, particularly when it comes to naming molecules that exhibit stereoisomerism. The International Union of Pure and Applied Chemistry (IUPAC) provides a systematic approach to naming all molecules, ensuring that each has a unique identifier. This becomes especially important for stereoisomers, which are molecules that differ in spatial arrangement due to the presence of chiral centers.
To effectively name these stereoisomers, we utilize the Cahn-Ingold-Prelog nomenclature system, commonly referred to as the R and S naming system. This method allows us to distinguish between different configurations of chiral centers in a straightforward manner. While the full name may seem complex, in practice, you can simply refer to it as the R and S system, which is widely recognized in the field.
The process of determining the R or S configuration involves a series of steps that can be broken down into a five-step procedure. Each step will guide you through the identification and naming of chiral centers, ensuring that you can apply these rules effectively to various molecules. As we progress, you will learn how to implement this system, enhancing your understanding of stereochemistry and its implications in molecular behavior.