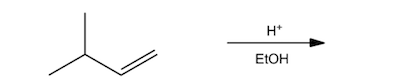Ethers are a class of organic compounds characterized by an oxygen atom connected to two alkyl or aryl groups. There are several methods for synthesizing ethers, each involving distinct reactions and mechanisms. Understanding these methods is crucial for mastering ether synthesis.
One common method for synthesizing ethers is through the Williamson Ether Synthesis. This reaction involves the nucleophilic substitution of an alkoxide ion with a primary alkyl halide. The general reaction can be represented as:
R1O- + R2Br → R1O-R2 + Br-
In this mechanism, the alkoxide ion acts as a nucleophile, attacking the electrophilic carbon of the alkyl halide, leading to the formation of the ether and the release of a halide ion. The reaction is most effective with primary alkyl halides to avoid steric hindrance and elimination reactions.
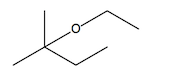
Another method for ether synthesis is the acid-catalyzed dehydration of alcohols. This process typically involves two alcohol molecules reacting in the presence of an acid catalyst, such as sulfuric acid. The reaction can be summarized as:
2 R-OH → R-O-R + H2O
In this mechanism, one alcohol molecule is protonated by the acid, forming a better leaving group. This activated alcohol can then undergo nucleophilic attack by another alcohol molecule, resulting in ether formation and the elimination of water.
Additionally, ethers can be synthesized through the alkylation of phenols. In this reaction, a phenol reacts with an alkyl halide in the presence of a base, leading to the formation of an ether. The general reaction is:
Ar-OH + R-X + Base → Ar-O-R + HX
Here, the phenoxide ion (Ar-O-) acts as a nucleophile, attacking the alkyl halide to form the ether and releasing a hydrogen halide.
Understanding these mechanisms and the conditions required for each reaction is essential for successfully synthesizing ethers in the laboratory. Each method has its advantages and limitations, making it important to choose the appropriate synthesis route based on the desired ether structure and available reagents.