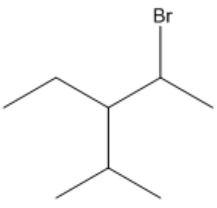
Alkyl halides are a class of organic compounds formed by substituting one or more hydrogen atoms in an alkane with halogen atoms. The naming convention for alkyl halides involves using specific prefixes for the halogens: fluorine is denoted as fluoro, chlorine as chloro, bromine as bromo, and iodine as iodo. These prefixes are added before the root name of the alkane to indicate the presence and position of the halogen substituents.
When numbering the carbon chain in alkyl halides, it is important to note that halogens do not have priority over other substituents. This means that the numbering should start from the end of the chain that is closest to any substituent, regardless of whether it is a halogen or another group. This approach ensures that the substituents receive the lowest possible numbers, following the same rules applied to alkanes.
For example, if you have a carbon chain with both a halogen and an alkyl group, you would number the chain starting from the end that is closest to any substituent, not just the halogen. This method maintains consistency in naming and helps avoid confusion when identifying the structure of the compound.
To practice, try drawing out an alkyl halide structure and apply these naming conventions. Once you have completed your drawing and determined the correct name, you can check your understanding by comparing it with additional resources or exercises.