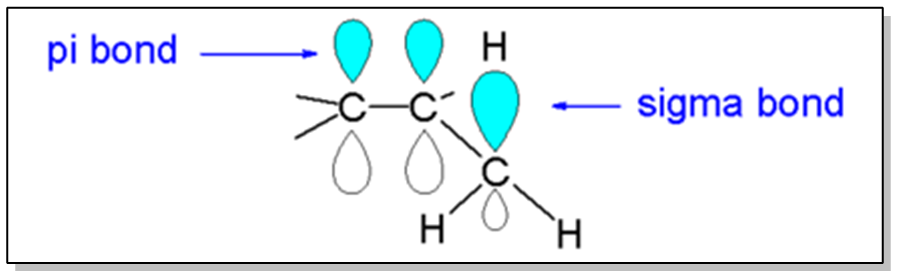
Alkenes exhibit stability through a phenomenon known as hyperconjugation, which is also a stabilizing force for carbocations. This concept is crucial when analyzing the stability of alkenes, as it relates to the presence of adjacent sigma bonds that can interact with the pi bond formed by overlapping p orbitals. The more substituents (R groups) present around the double bond, the greater the stabilization due to hyperconjugation, as these groups can share electron density with the pi bond.
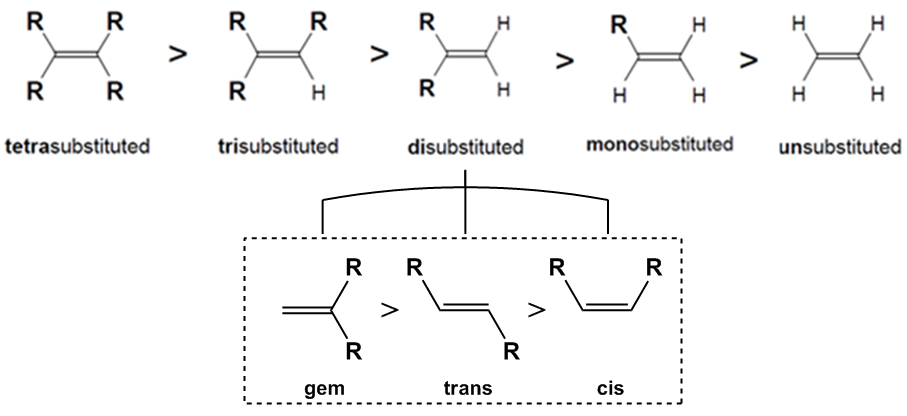
The stability of alkenes can be ranked based on the number of substituents around the double bond. The trend in stability is as follows: tetra-substituted alkenes are the most stable, followed by tri-substituted, di-substituted, mono-substituted, and finally unsubstituted alkenes, which lack any stabilizing groups and are therefore the least stable.
When considering di-substituted alkenes, there are different configurations that can affect stability. These configurations include:
- cis: Both substituents are on the same side of the double bond, leading to steric hindrance and reduced stability.
- trans: Substituents are on opposite sides, allowing for more spatial separation and increased stability.
- geminal: Both substituents are attached to the same carbon atom, which is referred to as geminal (from the Latin word for twins). This configuration is surprisingly the most stable due to unique interactions, despite being less intuitive than the trans configuration.
In summary, the stability of alkenes is significantly influenced by the number and arrangement of substituents around the double bond. Understanding these concepts is essential for predicting the behavior and reactivity of alkenes in organic chemistry.