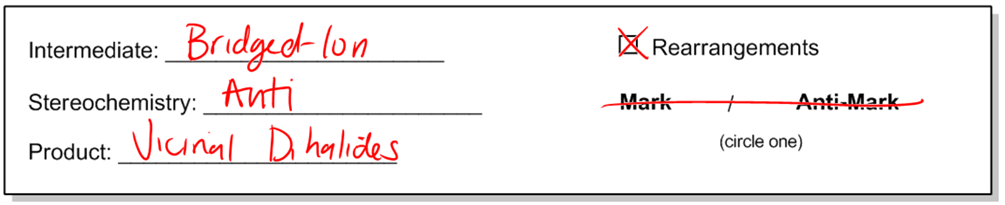
Halogenation is a significant addition reaction involving the transformation of a double bond by the addition of a diatomic halogen, resulting in the formation of anti vicinal dihalides. This process is characterized by a specific mechanism that includes the formation of a bridged ion intermediate, which is a common feature in various addition reactions.
The stereochemistry of halogenation is crucial, as it yields anti dihalides. This means that the two halogen atoms are added to opposite sides of the double bond, leading to a configuration where the substituents are positioned on adjacent carbon atoms, referred to as vicinal. The term "vicinal" indicates that the substituents are located on the first and second carbon atoms of the chain, hence the designation of 1,2-dihalides.
Importantly, the halogenation mechanism does not involve rearrangements, as there are no carbocation intermediates formed during the reaction. This simplifies the process, as the addition of two identical halogen atoms eliminates the need to consider Markovnikov's rule, which typically applies to reactions involving different substituents.
In terms of solvents, nonpolar solvents such as carbon tetrachloride (CCl4) or dichloromethane (CH2Cl2) are often used in halogenation reactions. These solvents are inert and do not participate in the reaction, serving merely to facilitate the process without influencing the outcome.
Overall, halogenation is a straightforward addition reaction that effectively converts alkenes into vicinal dihalides through a well-defined mechanism, characterized by anti stereochemistry and the absence of rearrangements.