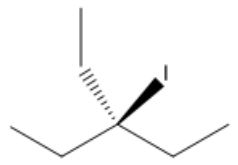
In elimination reactions, the process involves the removal of beta hydrogens, leading to the formation of double bonds. The fundamental concept of elimination can be summarized as the transformation of two sigma bonds into one pi bond. This reaction typically occurs when a leaving group is present alongside a hydrogen atom bonded to a beta carbon. The challenge arises from the presence of multiple beta hydrogens, as the choice of which hydrogen to remove can result in different products.
To understand the potential outcomes of an elimination reaction, it is essential to identify the number of non-equivalent beta carbons. A beta carbon is defined as the carbon atom adjacent to the alpha carbon, which is directly bonded to the leaving group. Each unique beta carbon that possesses at least one hydrogen atom represents a distinct product that can be formed during the elimination process. Therefore, the maximum number of products typically generated from a single elimination reaction is three, corresponding to the maximum number of unique beta carbons available.
To determine the number of possible products, one must count the non-equivalent beta carbons and assess whether they have hydrogen atoms available for removal. If a beta carbon has at least one hydrogen, it can contribute to the formation of a unique product. In some cases, there may be only one viable product if only one beta carbon has a hydrogen, while in other scenarios, up to three products may be possible based on the unique beta carbons present.
As you practice identifying the number of products from elimination reactions, focus on analyzing the beta carbons and their attached hydrogens. This approach will enhance your understanding of the reaction mechanisms and the diversity of products that can arise from a single elimination reaction.