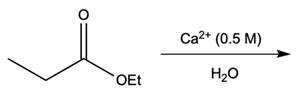
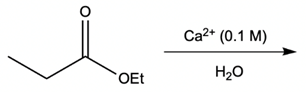
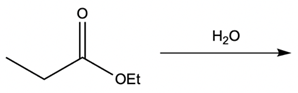
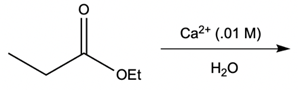
Metal ion catalysis plays a crucial role in enhancing reaction rates through the interaction of metal ions, typically those with a +2 or +3 charge, with electron-rich atoms. These metal ions act as catalysts by forming complexes that facilitate chemical reactions in two primary ways: metal ion coordination and water activation.
In metal ion coordination, the metal ion behaves similarly to a proton (H+). It can either increase the reactivity of a carbonyl carbon towards nucleophilic attack or stabilize a leaving group during a reaction. For instance, when a metal ion coordinates with the oxygen of a carbonyl group, it creates a bond that makes the carbonyl carbon more susceptible to nucleophilic attack. This interaction can be represented as a dashed bond between the metal and the oxygen, resulting in a partially positive charge on both the metal and the oxygen. Consequently, this facilitates the nucleophile's approach, allowing it to attack the carbonyl carbon and displace the existing bond.
Additionally, the metal ion can stabilize the leaving group. When a leaving group, such as an ethoxy group (OEt), is coordinated to the metal, it becomes a better leaving group due to the partial positive charge imparted by the metal. This stabilization allows the leaving group to depart more easily, promoting the overall reaction.
Water activation is another significant aspect of metal ion catalysis. Here, the metal ion enhances the hydrolysis of water, converting it into a stronger nucleophile. Hydrolysis involves the dissociation of water into H+ and OH- ions. Although the equilibrium favors the formation of water, a small amount of metal hydroxide complex can be generated, which is essential for biological reactions. Under physiological pH (approximately 7.4), a full hydroxide ion is not prevalent; thus, the metal hydroxide complex serves as a more reactive nucleophile. This transformation allows for nucleophilic addition and substitution reactions, which are vital in various biochemical processes.
In summary, metal ion catalysis encompasses the coordination of metal ions to enhance nucleophilicity and stabilize leaving groups, as well as the activation of water to form reactive metal hydroxide complexes. These mechanisms are fundamental in facilitating numerous chemical reactions, particularly in biological systems.