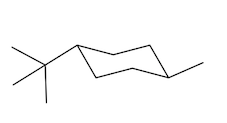
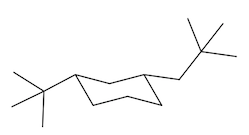
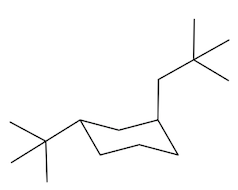
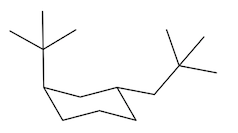
Equatorial preference in cyclohexane structures is crucial for understanding molecular stability. In cyclohexane, substituents can occupy two types of positions: axial and equatorial. The axial positions are oriented straight up and down, while the equatorial positions extend outward, resembling the equator of a globe. This spatial arrangement significantly impacts steric interactions, particularly when larger substituents are present.
When bulky groups, such as tert-butyl, are placed in axial positions, they experience increased torsional strain due to close proximity to other axial hydrogens. This crowding leads to unfavorable interactions, making the axial configuration less stable. In contrast, substituents in equatorial positions have more room to spread out, resulting in a more stable arrangement. For instance, a tert-butyl group prefers the equatorial position, where it can minimize steric hindrance and torsional strain.
When a cyclohexane ring undergoes a chair flip, the positions of substituents switch: axial groups become equatorial and vice versa. This flipping process allows bulky substituents to transition to the more stable equatorial position. Statistically, over 99% of a compound with a bulky substituent will exist in the equatorial configuration, highlighting the significant stability advantage it offers compared to the axial configuration, which accounts for less than 1% of the compound's existence.
In summary, understanding the preference for equatorial positions in cyclohexane derivatives is essential for predicting molecular behavior and stability. The axial positions are associated with increased steric strain, while equatorial positions provide a more favorable environment for larger substituents, leading to greater overall stability in the molecular structure.