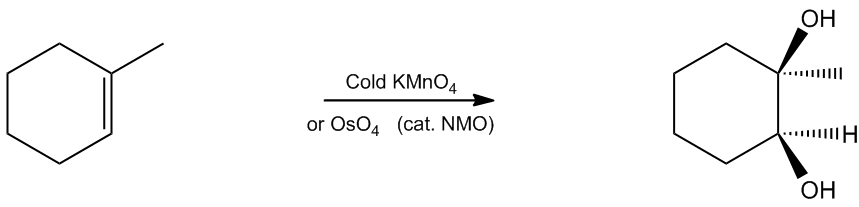
In the study of organic chemistry, one important reaction to understand is syn-vicinal dihydroxylation, which involves the addition of two alcohols to the same double bond in a single step. This reaction utilizes two key reagents: potassium permanganate (KMnO4) and osmium tetroxide (OsO4). Both of these reagents are effective at adding oxygen atoms to double bonds, resulting in the formation of diols, which are compounds containing two alcohol functional groups.
The term "vicinal" refers to the positioning of the alcohols; they are adjacent to each other on the carbon chain, specifically in a 1,2 relationship. The addition occurs in a syn manner, meaning that the two alcohols are added to the same side of the double bond, resulting in a cis configuration.
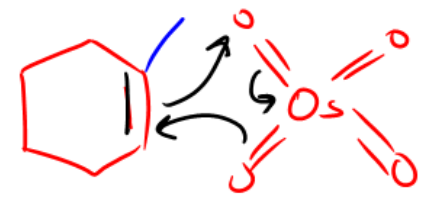
To visualize the mechanism, one can think of the reagents as "spaceships" that approach the double bond. When these reagents interact with the double bond, they effectively "attack" it, adding an oxygen atom to each carbon involved in the double bond. This results in the formation of a cyclic intermediate, where the double bond temporarily forms a ring structure with the reagent. Following this, the reaction yields the final product, a diol, as the reagents leave the reaction site.
While the complete mechanism of syn-vicinal dihydroxylation is not necessary to memorize, understanding the initial step can be beneficial. In this step, the double bond interacts with the osmium tetroxide, leading to the formation of a cyclic compound where the double bond is converted into two new bonds with oxygen. Ultimately, this reaction is a straightforward way to convert alkenes into diols, showcasing the utility of these reagents in organic synthesis.