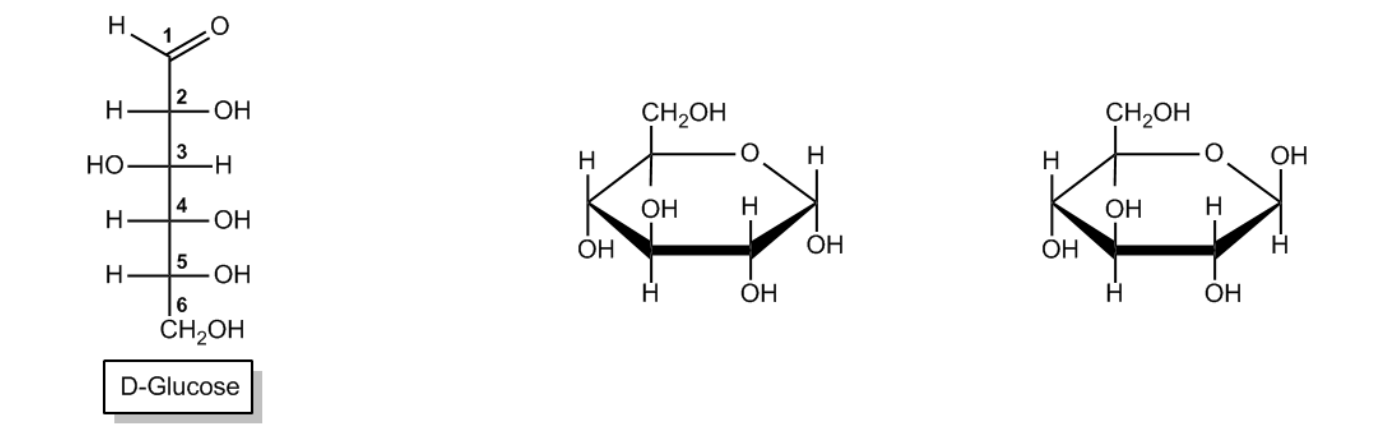
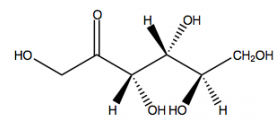
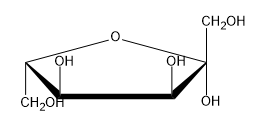
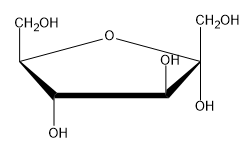
The Haworth projection is a crucial representation in sugar chemistry, particularly for visualizing monosaccharides in their cyclic forms. These projections simplify the structure of sugars by depicting them as planar rings, even though the actual conformation is a puckered chair shape, similar to cyclohexane. This simplification makes it easier to draw and understand the molecular structure.
When converting a monosaccharide chain into a Haworth projection, the process mirrors that of cyclohexane. The anomeric carbon, which is pivotal in determining the sugar's properties, is always positioned at the far right in the projection. This carbon corresponds to the first carbon in the numbering sequence, which proceeds clockwise around the ring. For example, in a typical pyranose ring, the carbons are numbered as follows: 1 (anomeric), 2, 3, 4, 5, and 6. This numbering convention applies equally to furanose rings, where the anomeric carbon remains on the right.
To identify anomers, one must observe the orientation of the hydroxyl groups on the anomeric carbon. In the case of D-glucose, if the hydroxyl group on the anomeric carbon is oriented down, it is classified as the alpha anomer; if it is oriented up, it is the beta anomer. The stereochemistry of D-glucose dictates that the hydroxyl group on carbon 6 is positioned upwards, consistent with the D stereoisomer designation.
Understanding anomeric equilibrium is also essential. For D-glucose, the beta anomer is favored over the alpha anomer in solution, indicating a preference for the configuration where the hydroxyl group on the anomeric carbon is in the equatorial position. This equilibrium can vary for different sugars, but for glucose, the beta form predominates.
In summary, the Haworth projection serves as an effective tool for representing monosaccharides, allowing for easy identification of anomers and understanding their equilibrium states. Mastery of this representation is fundamental for further studies in carbohydrate chemistry.