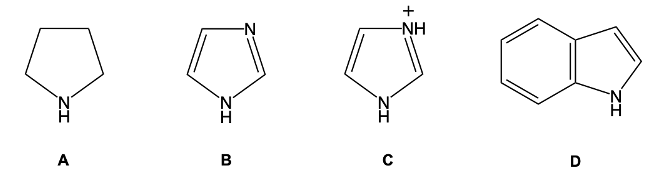
Aromatic heterocyclic amines exhibit unique weak basicity influenced by two primary factors: hybridization and aromaticity. Understanding these factors is crucial for grasping the acidic and basic properties of these compounds.
Hybridization plays a significant role in determining basicity. The basicity of a compound is inversely related to the s character of the orbital containing the lone pair of electrons. Specifically, the greater the s character, the lower the basicity. For instance, consider two heterocyclic compounds: one with an sp3 hybridized nitrogen and another with an sp2 hybridized nitrogen. The sp3 hybridized nitrogen has 25% s character, while the sp2 hybridized nitrogen has 33% s character.
In the case of the sp3 hybridized nitrogen, the lone pair is not involved in the aromatic system, making it less available for donation. This results in a higher pKb value, indicating weaker basicity. For example, the pKb for the sp3 hybridized compound is 4, while for the sp2 hybridized compound (like pyridine), the pKb is 8.8. This demonstrates that as the s character increases, the basicity decreases, leading to a higher pKb value.
In summary, the relationship between hybridization, s character, and basicity is essential for understanding the behavior of aromatic heterocyclic amines. The more s character present in the lone pair's orbital, the weaker the base becomes, as indicated by a higher pKb value.