The secondary protein structure is primarily defined by the hydrogen bonding that occurs between the backbone atoms of a protein. This structure arises from interactions between the amide hydrogen of one peptide bond and the carbonyl oxygen of another. In this context, the amide group, represented by the NH connection to a carbonyl, plays a crucial role in forming these hydrogen bonds.
To visualize this, consider two peptide chains: peptide chain 1 and peptide chain 2. The amide hydrogen from one chain can form a hydrogen bond with the carbonyl oxygen of the other chain. It is important to note that hydrogen bonding is an intermolecular force rather than a true bond, which is why these interactions are often depicted with dashed lines in structural representations.
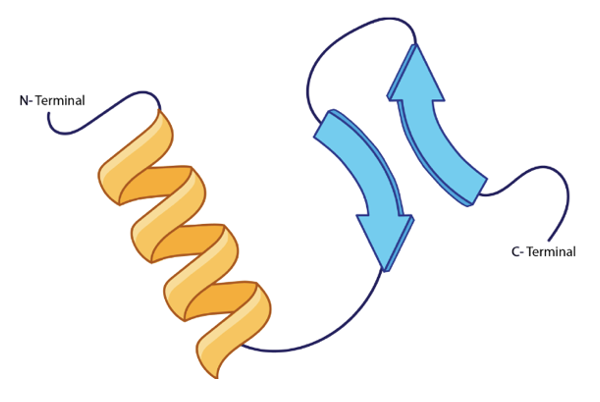
In the example, there are two distinct sites where hydrogen bonding occurs, contributing to the stability and formation of the secondary structure. The presence of R groups, which are side chains attached to the backbone, does not directly influence these hydrogen bonds unless they are specifically involved in interactions. Overall, the hydrogen bonding between amide and carbonyl groups is essential for establishing the secondary structure of proteins, such as alpha helices and beta sheets, which are fundamental to protein functionality.