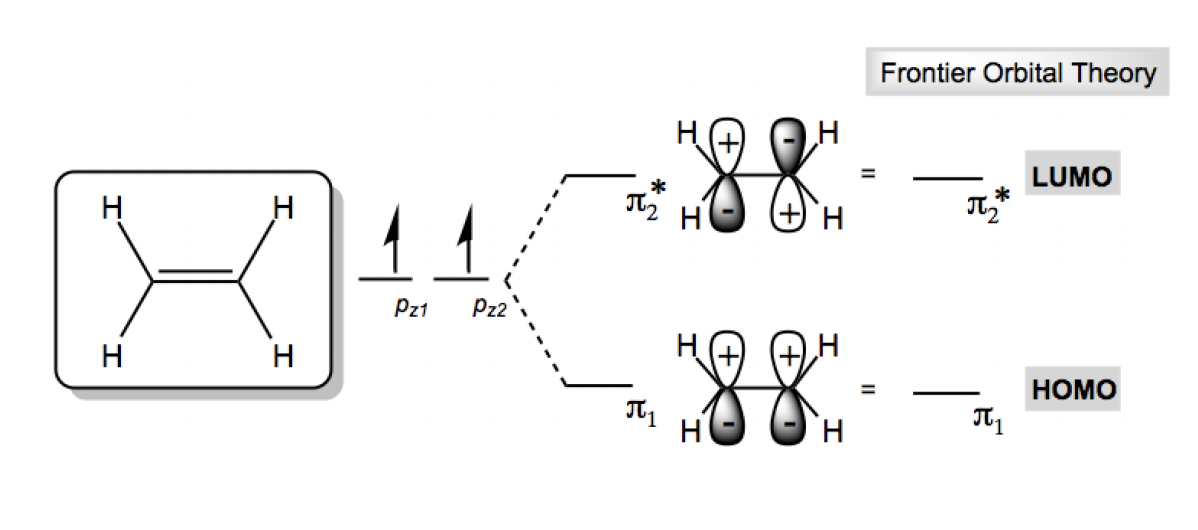
Frontier Molecular Orbital Theory (FMOT) is a crucial concept in organic chemistry that helps explain the reactivity of molecules. Understanding FMOT is foundational for grasping various chemical reactions. The key components of this theory are the Highest Occupied Molecular Orbital (HOMO) and the Lowest Unoccupied Molecular Orbital (LUMO).
The HOMO is defined as the molecular orbital with the highest energy that still contains electrons. Conversely, the LUMO is the molecular orbital with the lowest energy that does not contain any electrons. To illustrate this, consider the example of ethene (C2H4), a simple molecule that can help clarify these concepts.
In ethene, two atomic orbitals combine to form two molecular orbitals: a bonding orbital and an antibonding orbital. According to the principles of electron configuration, the two electrons in ethene will occupy the lowest energy bonding orbital, referred to as π1. This means that the HOMO for ethene is π1, which contains the two electrons. The LUMO, on the other hand, is the antibonding orbital, π2*, which is unoccupied and has no electrons.
In summary, for ethene, the HOMO is π1 with 2 electrons, while the LUMO is π2* with 0 electrons. As molecules become larger and more complex, identifying the HOMO and LUMO involves determining the boundary between filled and unfilled molecular orbitals. This understanding is essential for predicting how molecules will interact in chemical reactions.