Nucleophiles play a crucial role in organic chemistry, particularly in reactions involving electron donation. According to the Lewis definition, a nucleophile is characterized as a good electron donor, which is essential for its function in chemical reactions. In contrast, a base is defined by the Bronsted-Lowry theory as a good proton acceptor. While there is often overlap between nucleophilicity and basicity, they are not always synonymous. Understanding the distinctions between these two concepts is vital for grasping the nuances of chemical reactivity.
The strength of a nucleophile can be influenced by several factors. The first rule to remember is that negatively charged species are generally stronger nucleophiles than their neutral counterparts. This is a foundational concept that highlights the relationship between charge and nucleophilicity.
The second rule pertains to the steric hindrance of the nucleophile. Bulkier nucleophiles tend to be more basic but less nucleophilic. This is because a bulky nucleophile has difficulty approaching electrophiles due to its size, which hinders its ability to donate electrons effectively. However, it may still be proficient at accepting protons, as protons are often located at the edges of molecules, making them more accessible.
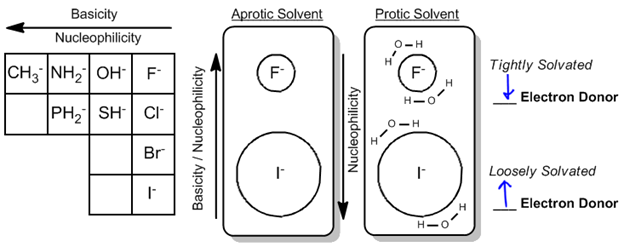
The third rule indicates that basicity and nucleophilicity typically trend in the same direction. As one moves across the periodic table, nucleophiles become stronger as electronegativity decreases and as one moves up the table, where smaller atoms are better electron donors due to their reduced size and increased ability to donate electrons.
However, an important exception arises in the presence of protic solvents, which can hydrogen bond and solvate ions. In protic solvents, smaller anions like fluoride are more effectively solvated, meaning they are surrounded by solvent molecules that hinder their ability to donate electrons. Conversely, larger anions like iodide are less tightly solvated, allowing them to act as better electron donors despite being weaker nucleophiles in a non-protic environment. Thus, in protic solvents, iodide can emerge as the best nucleophile, while fluoride may be less effective.
In summary, understanding the behavior of nucleophiles in different solvent environments is essential for predicting their reactivity in various chemical contexts. Mastery of these concepts will aid in answering conceptual questions commonly encountered in examinations and deepen comprehension of organic reaction mechanisms.