The Birch reduction is a specific type of reduction reaction that transforms benzene into an isolated diene, particularly an isolated cyclohexadiene when starting with unsubstituted benzene. This reaction utilizes elemental sodium in conjunction with an amine and an alcohol, typically ethanol or tert-butanol, to facilitate the process. The key outcome of the Birch reduction is the formation of two double bonds that are positioned 1,4 relative to each other on the cyclohexane ring.
The mechanism of the Birch reduction is analogous to the dissolving metal reduction, which is familiar from organic chemistry studies involving alkynes. It begins with elemental sodium, which possesses a single electron. This electron is donated to one of the carbon atoms in the benzene ring, resulting in the formation of a radical anion. This intermediate consists of a radical at the top, double bonds on both sides, and a carbanion at the bottom, which is created by the ionization of a double bond into a lone pair.
In the next step, ethanol acts as a protonating agent, where the carbanion captures a hydrogen atom from ethanol, leading to the formation of a new radical. This process is repeated with another equivalent of elemental sodium, generating another carbanion intermediate. The reaction continues until the final product, the isolated diene, is formed, characterized by two hydrogen atoms on the top and bottom of the cyclohexadiene structure.
It is important to note that the Birch reduction requires sufficient equivalents of alcohol to ensure the reaction proceeds to completion. The regioselectivity of the reaction is also a critical aspect to consider, as it influences the final structure of the isolated diene. Understanding the mechanism and the role of each reagent is essential for mastering the Birch reduction and its applications in organic synthesis.



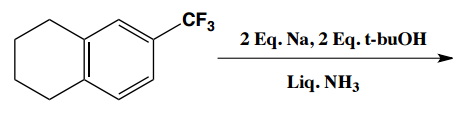


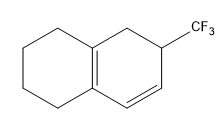

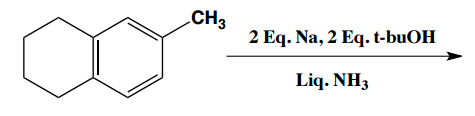
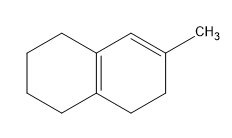


 Birch reduction specific examples
Birch reduction specific examples
