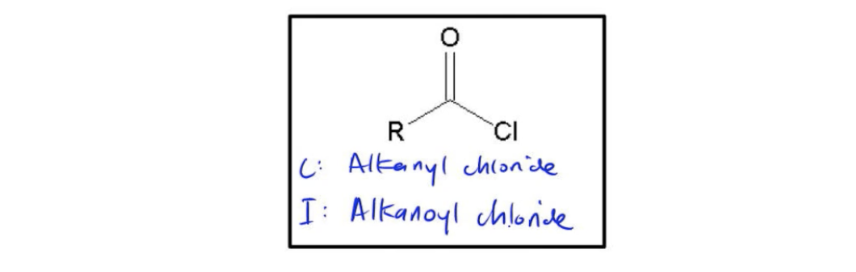
Acid chlorides, also known as acyloids, are named using two different conventions: common naming and IUPAC naming. Understanding the distinction between these two methods is essential for accurately identifying these compounds.
To illustrate the naming process, consider a three-carbon acid chloride. For the common name, you start with the corresponding carboxylic acid. In this case, the three-carbon carboxylic acid is propionic acid. To convert this to the common name for the acid chloride, you replace the "ic acid" ending with "yl chloride." Thus, propionic acid becomes propanyl chloride.
In contrast, the IUPAC naming convention begins with the alkane name corresponding to the carbon chain. For a three-carbon chain, the base name is propane. To form the IUPAC name for the acid chloride, you remove the "e" from propane and add the suffix "oyl chloride." Therefore, propane becomes propanoyl chloride.
In summary, the common name for a three-carbon acid chloride is derived from the carboxylic acid by replacing "ic acid" with "yl chloride," while the IUPAC name is formed by modifying the alkane name by removing the "e" and adding "oyl chloride." This systematic approach to naming acid chlorides is crucial for effective communication in organic chemistry.