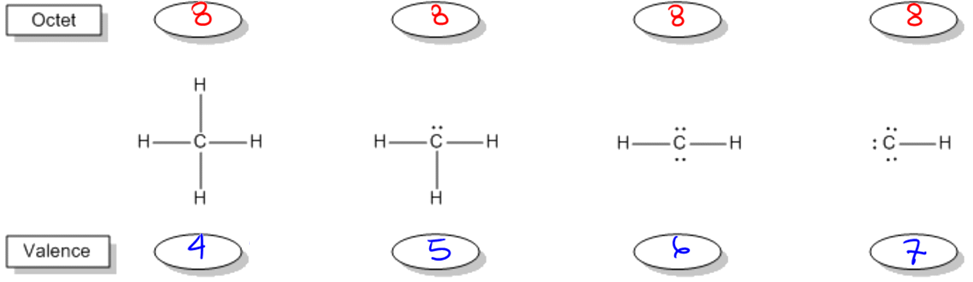
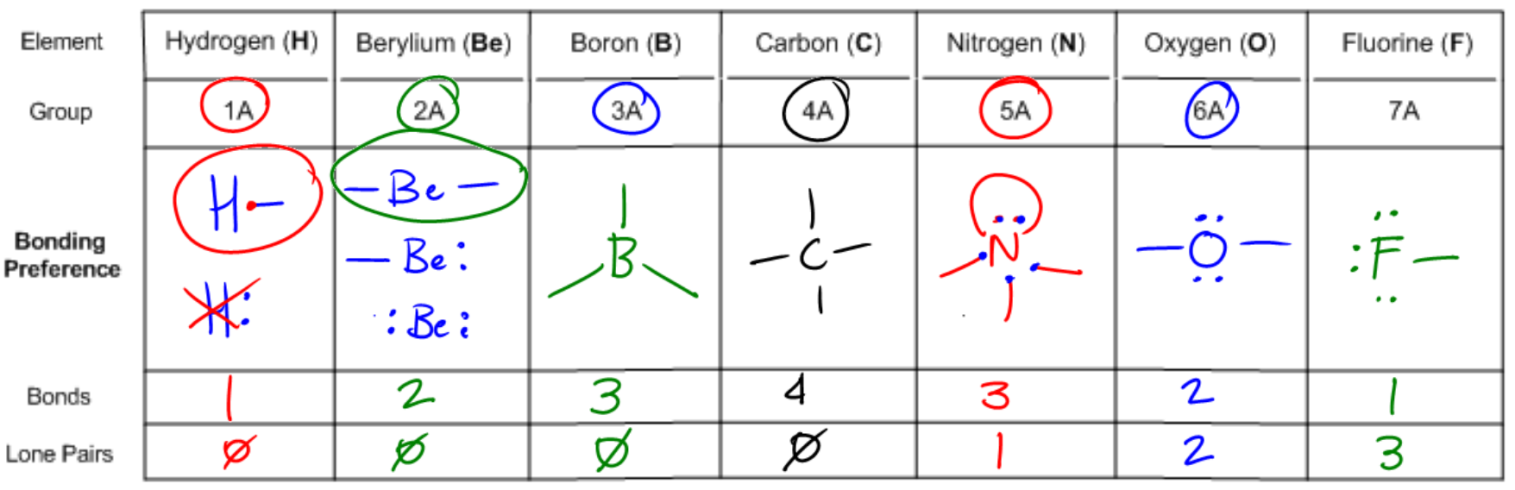
Understanding bonding preferences is crucial in chemistry, particularly when studying how atoms interact and form compounds. At the heart of this concept lies the octet rule, which states that atoms tend to form bonds until they are surrounded by eight valence electrons, achieving a stable electron configuration. Valence electrons are the electrons that an atom possesses in its outer shell, and they play a significant role in determining how many bonds an atom can form.
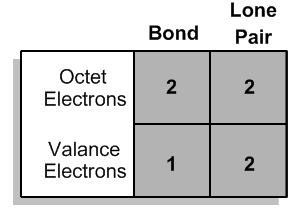
Atoms can either share electrons through bonds or retain them as lone pairs. Both shared and lone electrons contribute to the total count of octet electrons, but they are counted differently when determining valence electrons. Specifically, an atom will count each lone electron as one valence electron and will count only one electron for each bond it forms. This can be summarized simply: each lone pair (represented as a dot) counts as one electron, and each bond (represented as a stick) also counts as one electron.
To illustrate, if an atom has three lone electrons and forms two bonds, its total valence electron count would be five (three from lone electrons and two from bonds). This understanding allows chemists to predict the bonding behavior of different elements based on their valence electron configurations. By mastering these principles, students can confidently determine the bonding preferences of various atoms, leading to a deeper comprehension of molecular structures and stability.