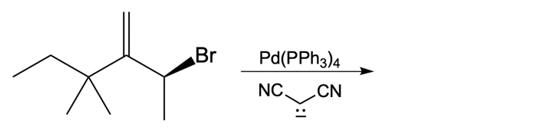
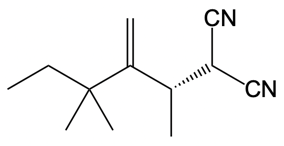
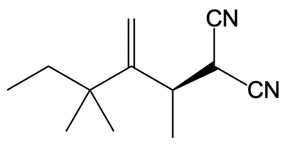
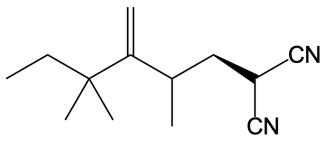
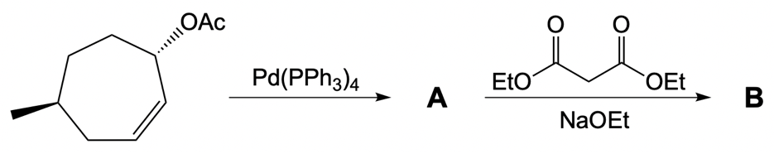
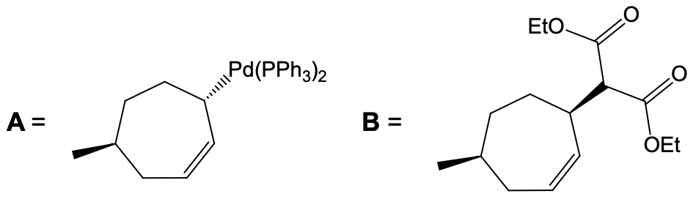
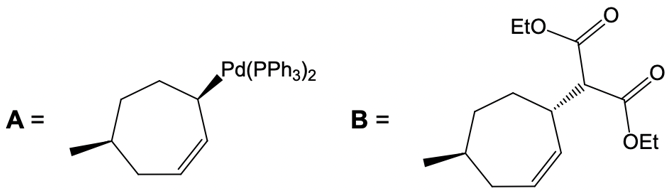
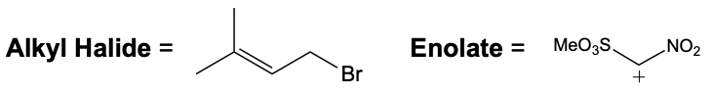
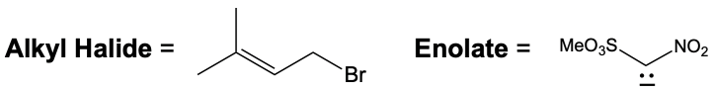
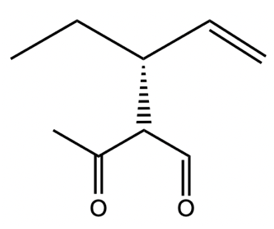
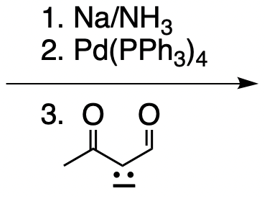
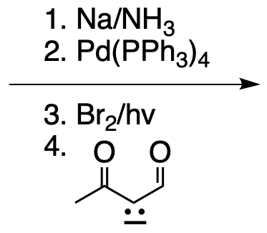
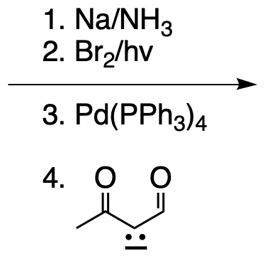
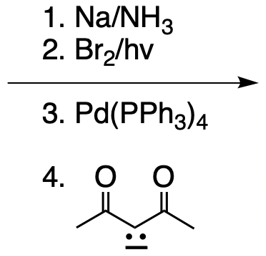
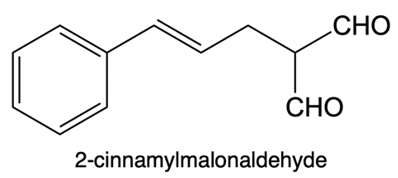
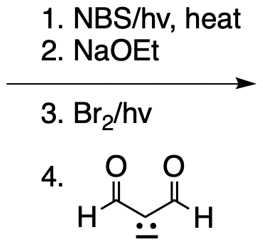
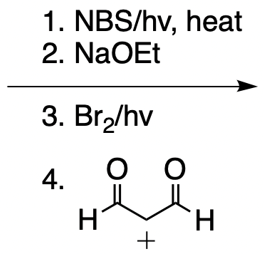
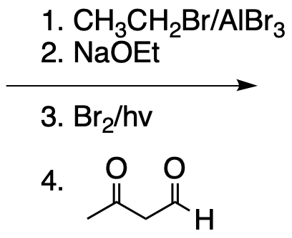
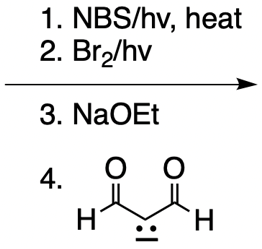
Catalytic allylic alkylation involves the coupling of an allylic carbon with an enolate, a key concept in organic chemistry. An enolate is formed from an alpha carbon and plays a crucial role in this reaction. The process is characterized by its high stereoselectivity, favoring the production of one enantiomer over another. This reaction proceeds through a double SN2 mechanism, maintaining the retention of the R/S configuration of the starting compound.
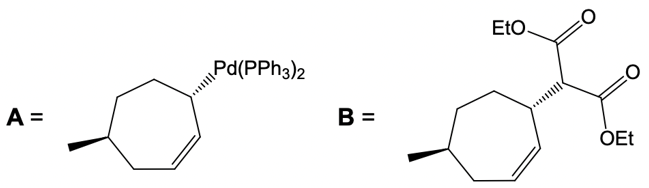
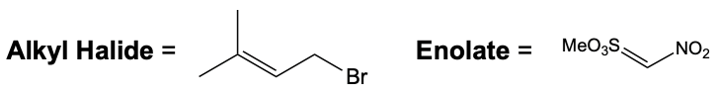
In this reaction, the allylic carbon is chiral, and the leaving group (denoted as X) can be an ester or a halogen such as chlorine, bromine, or iodine. The palladium catalyst, which can be represented as Pd with ligands like triphenylphosphine (PPh3) or palladium(II) chloride (PdCl2), facilitates the reaction. The presence of the palladium catalyst is essential as it induces inversion of configuration at the chiral center, transforming the bond from a dashed to a wedged configuration.
After the initial SN2 reaction with the palladium catalyst, the nucleophile, which is an enolate in this case, interacts with the allylic carbon. This second SN2 reaction results in the inversion back to the original configuration, restoring the dashed bond with the nucleophile attached. The versatility of the leaving groups, including esters, enhances the efficiency of this catalytic process, making it a valuable method in synthetic organic chemistry.
Overall, catalytic allylic alkylation exemplifies the intricate interplay between stereochemistry and reaction mechanisms, showcasing how catalysts can influence the outcome of chemical reactions while maintaining specific configurations.