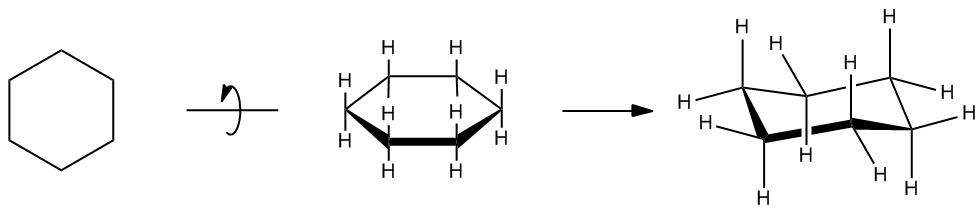
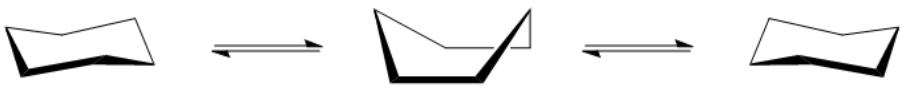
In the study of cycloalkanes, particularly cyclohexane, it is essential to understand the concept of molecular geometry and strain. While it is common to represent cyclohexane as a flat, planar structure, the reality is that it adopts a puckered conformation. This puckered shape is crucial for minimizing both torsional strain and ring strain.
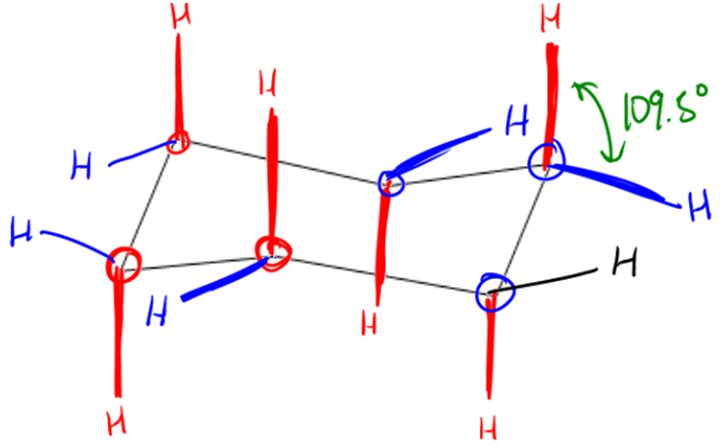
Torsional strain arises when atoms, such as hydrogen atoms in cyclohexane, are eclipsed, leading to increased energy due to repulsion. In a planar cyclohexane, the hydrogen atoms would overlap, resulting in significant torsional strain. Additionally, ring strain occurs when the bond angles deviate from their ideal values. For cyclohexane, the ideal bond angle is approximately 109.5 degrees, but a planar structure would force these angles to be around 120 degrees, further contributing to ring strain.
To alleviate these strains, cyclohexane adopts a chair conformation, which is considered the most stable form of cycloalkane. In the chair conformation, the hydrogen atoms are positioned in such a way that they are staggered rather than eclipsed, effectively reducing torsional strain. Furthermore, the bond angles in the chair conformation are close to the ideal tetrahedral angle of 109.5 degrees, minimizing ring strain. This unique structure allows cyclohexane to exist with virtually no torsional or ring strain, making it an excellent example of molecular stability in organic chemistry.